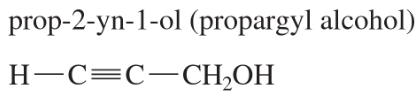Back
BackProblem 1
Draw structural formulas of at least two alkynes of each molecular formula.
1. C6H10
2. C8H12
3. C7H8
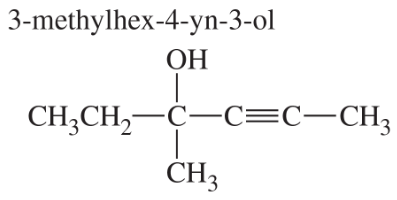
Problem 1a
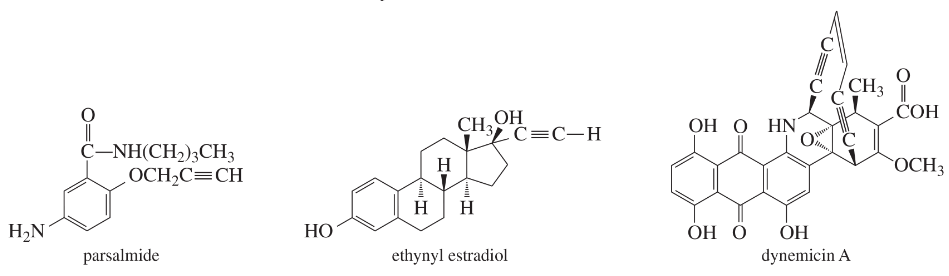
Count the elements of unsaturation in parsalmide, ethynyl estradiol, and dynemicin A.
Problem 2a
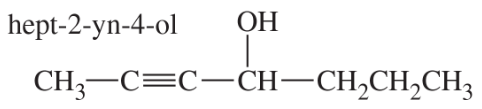
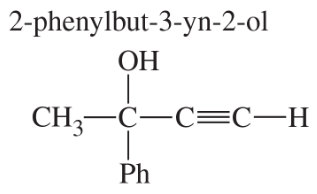
For each molecular formula, draw all the isomeric alkynes, and give their IUPAC names. Circle the acetylenic hydrogen of each terminal alkyne.
(a) C5H8 (three isomers)
Problem 2b
For each molecular formula, draw all the isomeric alkynes, and give their IUPAC names. Circle the acetylenic hydrogen of each terminal alkyne.
(b) C6H10 (seven isomers)
Problem 3
What reaction would acetylene likely undergo if it were kept at 1500°C for too long?
Problem 4
The boiling points of hex-1-ene (64 °C) and hex-1-yne (71 °C) are sufficiently close that it is difficult to achieve a clean separation by distillation. Show how you might use the acidity of hex-1-yne to remove the last trace of it from a sample of hex-1-ene.
Problem 5a,b,c
Predict the products of the following acid–base reactions, or indicate if no significant reaction would take place.
a. H—C≡C—H + NaNH2
b. H—C≡C—H + CH3Li
c. H—C≡C—H + NaOCH3
Problem 6
Solved Problem 9-1 showed the synthesis of dec-3-yne by adding the hexyl group first, then the ethyl group. Show the reagents and intermediates involved in the other order of synthesis of dec-3-yne, by adding the ethyl group first and the hexyl group last.
Problem 7a,b,c
Show how you might synthesize the following compounds, using acetylene and any suitable alkyl halides as your starting materials. If the compound given cannot be synthesized by this method, explain why.
a. hex-1-yne
b. hex-2-yne
c. hex-3-yne
Problem 7d,e,f
Show how you might synthesize the following compounds, using acetylene and any suitable alkyl halides as your starting materials. If the compound given cannot be synthesized by this method, explain why.
d. 4-methylhex-2-yne
e. 5-methylhex-2-yne
f. cyclodecyne
Problem 8a
Show how you would synthesize each compound, beginning with acetylene and any necessary additional reagents
(a)
Problem 8b
Show how you would synthesize each compound, beginning with acetylene and any necessary additional reagents
(b)
Problem 8c,d
Show how you would synthesize each compound, beginning with acetylene and any necessary additional reagents
(c)
(d)
Problem 9
Show how you would synthesize 2-phenylhex-3-yn-2-ol, starting with acetophenone (PhCOCH3) and any other reagents you need. ("2-ol" means there is an OH group on C2.)
Problem 10
When 2,2-dibromo-1-phenylpropane is heated overnight in fused KOH at 200 C, the major product is a foul-smelling compound of formula C9H8. Propose a structure for this product, and give a mechanism to account for its formation.
Problem-Solving Hint:

Problem 11
When 2,2-dibromo-1-phenylpropane is heated overnight with sodium amide at 150°C, the major product (after addition of water) is a different foul-smelling compound of formula C9H8. Propose a structure for this product, and give a mechanism to account for its formation.
Problem 12a,b
Show how you would convert
a. oct-3-yne to cis-oct-3-ene.
b. pent-2-yne to trans-pent-2-ene.
Problem 12c
Show how you would convert
c. cis-cyclodecene to trans-cyclodecene.
Problem 12d
Show how you would convert
d. but-1-yne to cis-hex-3-ene.
Problem 13
The fragrance of (Z)-1-phenylhex-2-en-1-ol resembles that of roses, with a delicate citrus edge. Show how you would synthesize this compound from benzaldehyde (PhCHO) and any other reagents you need.
Problem 14
In the addition of just 1 mole of bromine to 1 mole of hex-1-yne, should the hex-1-yne be added to a bromine solution or should the bromine be added to the hex-1-yne? Explain your answer.
Problem 15
Propose a mechanism for the entire reaction of pent-1-yne with 2 moles of HBr. Show why Markovnikov's rule should be observed in both the first and second additions of HBr.
Problem 16a,b
Predict the major product(s) of the following reactions:
a. phenylacetylene + 2 HBr
b. hex-1-yne + 2 HCl
Problem 16c,d
Predict the major product(s) of the following reactions:
c. cyclooctyne + 2 HBr
d. *hex-2-yne + 2 HCl
Problem 17
Propose a mechanism for the reaction of pent-1-yne with HBr in the presence of peroxides. Show why anti-Markovnikov orientation results.
Problem 18a,b,c
Show how hex-1-yne might be converted to
a. 1,2-dichlorohex-1-ene.
b. 1-bromohex-1-ene.
c. 2-bromohex-1-ene.
Problem 18e
Show how hex-1-yne might be converted to
e. 2-bromohexane.
Problem 18f
Show how hex-1-yne might be converted to
f. 2,2-dibromohexane.
Problem 19
When pent-2-yne reacts with mercuric sulfate in dilute sulfuric acid, the product is a mixture of two ketones. Give the structures of these products, and use mechanisms to show how they are formed.
Problem 20a
The hydroboration–oxidation of internal alkynes produces ketones.
a. When hydroboration–oxidation is applied to but-2-yne, a single pure product is obtained. Determine the structure of this product, and show the intermediates in its formation.