Back
BackProblem 5a,b,c
All of the following names are incorrect or incomplete. In each case, draw the structure (or a possible structure) and name it correctly.
a. 3-ethyl-4-methylpentane
b. 2-ethyl-3-methylpentane
c. 3-dimethylhexane
Problem 5d,e,f
All of the following names are incorrect or incomplete. In each case, draw the structure (or a possible structure) and name it correctly.
d. 4-isobutylheptane
e. 2-bromo-3-ethylbutane
f. 2,3-diethyl-5-isopropylheptane
Problem 6a
Give structures and names for
a. the five isomers of C6H14
Problem 7a
Draw the structures of the following groups, and give their more common names.
a. the (1-methylethyl) group
b. the (2-methylpropyl) group
c. the (1-methylpropyl) group
d. the (1,1-dimethylethyl) group
e. the (3-methylbutyl) group, sometimes called the 'isoamyl' group
Problem 8a
Draw the structures of the following compounds.
a. 4-(1,1-dimethylethyl)octane
Problem 8b
Draw the structures of the following compounds.
b. 5-(1,2,2-trimethylpropyl)nonane
Problem 8c
Draw the structures of the following compounds.
c. 3,3-diethyl-4-(2,2-dimethylpropyl)octane
Problem 9
Without looking at the structures, give molecular formulas for the compounds: (a) 4-(1,1-dimethylethyl)octane and (b) 5-(1,2,2-trimethylpropyl)nonane. Use the names of the groups to determine the number of carbon atoms; then use the (2n + 2) rule.
Problem 10a
List each set of compounds in order of increasing boiling point.
a. hexane, octane, and decane
Problem 10b
List each set of compounds in order of increasing boiling point.
b. Octane, (CH3)3C—C(CH3)3 and CH3CH2C(CH3)2CH2CH2CH3
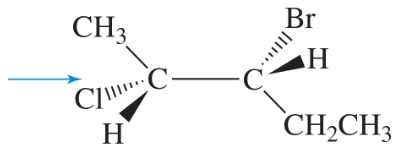
Problem 11a
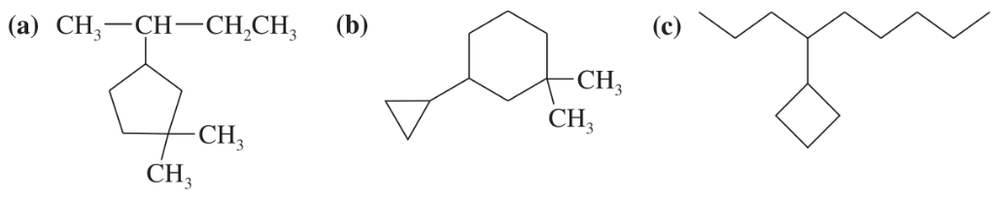
Draw Newman projections of the following molecules viewed from the direction of the blue arrows.
(a)
Problem 11b
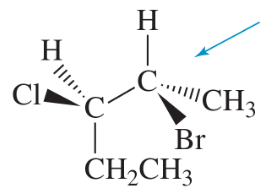
Draw Newman projections of the following molecules viewed from the direction of the blue arrows.
(b)
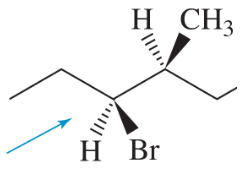
Problem 11c
Draw Newman projections of the following molecules viewed from the direction of the blue arrows.
(c)
Problem 12
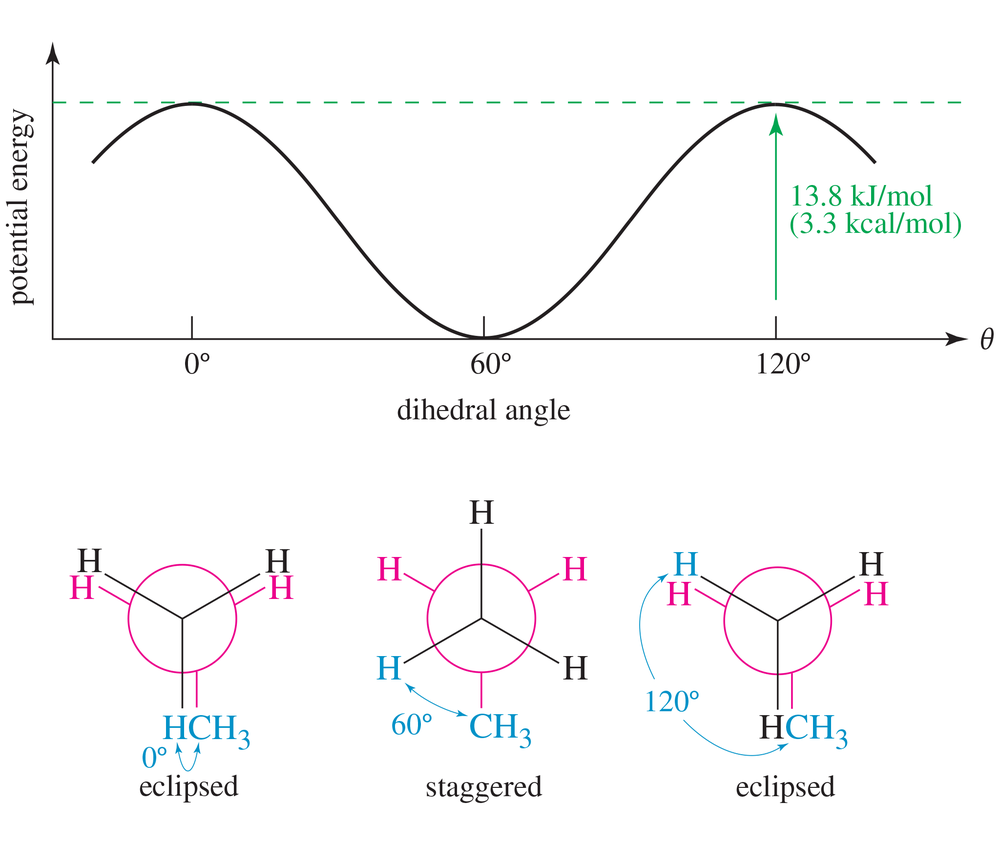
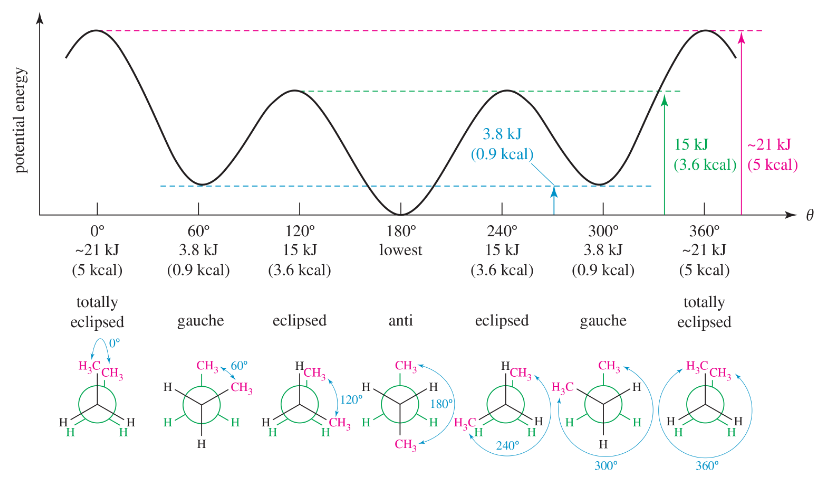
Draw a graph, similar to Figure 3-9, of the torsional strain of 2-methylpropane as it rotates about the bond between C1 and C2. Show the dihedral angle and draw a Newman projection for each staggered and eclipsed conformation.
Problem 13
Draw a graph, similar to Figure 3-11, of the torsional energy of 2-methylbutane as it rotates about the C2—C3 bond.
Problem 14
Draw a perspective representation of the most stable conformation of 3-methylhexane.
Problem 15a,b,c
Give IUPAC names for the following compounds.
Problem 16a,b
Draw the structure and give the molecular formula for each of the following compounds.
a. 1-ethyl-3-methylcycloheptane
b. isobutylcyclohexane
Problem 16c,d
Draw the structure and give the molecular formula for each of the following compounds.
c. cyclopropylcyclopentane
d. 3-ethyl-1,1-dimethylcyclohexane
Problem 16e,f
Draw the structure and give the molecular formula for each of the following compounds.
e. 3-ethyl-2,4-dimethylhexane
f. 1,1-diethyl-4-(3,3-dimethylbutyl)cyclohexane
Problem 16k,l
Draw the structure that corresponds with each name.
k. cyclobutylcyclohexane
l. cis-1-bromo-3-chlorocyclohexane
Problem 17a
Which of the following cycloalkanes are capable of geometric (cis-trans) isomerism? Draw the cis and trans isomers.
a. 3-ethyl-1,1-dimethylcyclohexane
Problem 17b
Which of the following cycloalkanes are capable of geometric (cis-trans) isomerism? Draw the cis and trans isomers.
b. 1-ethyl-3-methylcycloheptane
Problem 17c
Which of the following cycloalkanes are capable of geometric (cis-trans) isomerism? Draw the cis and trans isomers.
c. 1-ethyl-3-methylcyclopentane
Problem 19
The heat of combustion of cis-1,2-dimethylcyclopropane is larger than that of the trans isomer. Which isomer is more stable? Use drawings to explain this difference in stability.
Problem 20
trans-1,2-Dimethylcyclobutane is more stable than cis-1,2-dimethylcyclobutane, but cis-1,3-dimethylcyclobutane is more stable than trans-1,3-dimethylcyclobutane. Use drawings to explain these observations.
Problem 21
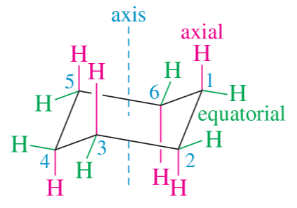
The cyclohexane chair shown in Figure 3-22 has the headrest to the right and the footrest to the left. Draw a cyclohexane chair with its axial and equatorial bonds, showing the headrest to the left and the footrest to the right.
Problem 22a
Draw 1,2,3,4,5,6-hexamethylcyclohexane with all the methyl groups
a. in axial positions.
Problem 22b
Draw 1,2,3,4,5,6-hexamethylcyclohexane with all the methyl groups
b. in equatorial positions.
Problem 23
Draw a Newman projection, similar to Figure 3-25 down the C1—C6 bond in the equatorial conformation of methylcyclohexane. Show that the equatorial methyl group is also anti to C5. (Using your models will help.)
<IMAGE>