Back
BackProblem 16k,l
Draw the structure that corresponds with each name.
k. cyclobutylcyclohexane
l. cis-1-bromo-3-chlorocyclohexane
Problem 17a
Which of the following cycloalkanes are capable of geometric (cis-trans) isomerism? Draw the cis and trans isomers.
a. 3-ethyl-1,1-dimethylcyclohexane
Problem 17b
Which of the following cycloalkanes are capable of geometric (cis-trans) isomerism? Draw the cis and trans isomers.
b. 1-ethyl-3-methylcycloheptane
Problem 17c
Which of the following cycloalkanes are capable of geometric (cis-trans) isomerism? Draw the cis and trans isomers.
c. 1-ethyl-3-methylcyclopentane
Problem 19
The heat of combustion of cis-1,2-dimethylcyclopropane is larger than that of the trans isomer. Which isomer is more stable? Use drawings to explain this difference in stability.
Problem 20
trans-1,2-Dimethylcyclobutane is more stable than cis-1,2-dimethylcyclobutane, but cis-1,3-dimethylcyclobutane is more stable than trans-1,3-dimethylcyclobutane. Use drawings to explain these observations.
Problem 21
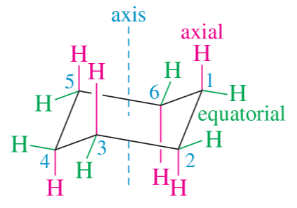
The cyclohexane chair shown in Figure 3-22 has the headrest to the right and the footrest to the left. Draw a cyclohexane chair with its axial and equatorial bonds, showing the headrest to the left and the footrest to the right.
Problem 22a
Draw 1,2,3,4,5,6-hexamethylcyclohexane with all the methyl groups
a. in axial positions.
Problem 22b
Draw 1,2,3,4,5,6-hexamethylcyclohexane with all the methyl groups
b. in equatorial positions.
Problem 23
Draw a Newman projection, similar to Figure 3-25 down the C1—C6 bond in the equatorial conformation of methylcyclohexane. Show that the equatorial methyl group is also anti to C5. (Using your models will help.)
<IMAGE>
Problem 24
Table 3-6 shows that the axial–equatorial energy difference for methyl, ethyl, and isopropyl groups increases gradually: 7.6, 7.9, and 8.8 kJ/mol (1.8, 1.9, and 2.1 kcal/mol). The tert-butyl group jumps to an energy difference of 23 kJ/mol (5.4 kcal/mol), over twice the value for the isopropyl group. Draw pictures of the axial conformations of isopropylcyclohexane and tert-butylcyclohexane, and explain why the tert-butyl substituent experiences such a large increase in axial energy over the isopropyl group.

Problem 25a
Draw the most stable conformation of
a. ethylcyclohexane.
Problem 25b
Draw the most stable conformation of
b. 3-isopropyl-1,1-dimethylcyclohexane.
Problem 25c
Draw the most stable conformation of
c. cis-1-tert-butyl-4-isopropylcyclohexane.
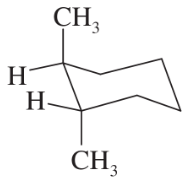
Problem 26c,d
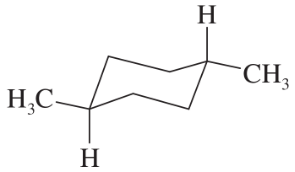
Name the following compounds. Remember that two up bonds are cis; two down bonds are cis; one up bond and one down bond are trans.
(c)
(d)
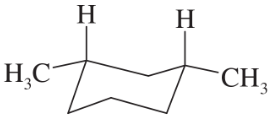
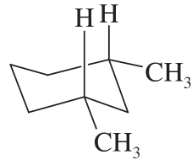
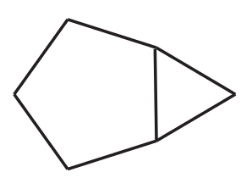
Problem 26e,f
Name the following compounds. Remember that two up bonds are cis; two down bonds are cis; one up bond and one down bond are trans.
(e)
(f)
Problem 27
a. Draw both chair conformations of cis-1,4-dimethylcyclohexane, and determine which conformer is more stable.
b. Repeat for the trans isomer.
c. Predict which isomer (cis or trans) is more stable.
Problem 28
Use your results from Problem 3-27 to complete the following table. Each entry shows the positions of two groups arranged as shown. For example, two groups that are trans on adjacent carbons (trans-1,2) must be both equatorial (e,e) or both axial (a,a).
Problem 29a,b
Draw the two chair conformations of each of the following substituted cyclohexanes. In each case, label the more stable conformation.
a. cis-1-ethyl-2-methylcyclohexane
b. trans-1,2-diethylcyclohexane
Problem 29c,d
Draw the two chair conformations of each of the following substituted cyclohexanes. In each case, label the more stable conformation.]
c. cis-1-ethyl-4-isopropylcyclohexane
d. trans-1-ethyl-4-methylcyclohexane
Problem 30a
Draw the most stable conformation of
a. cis-1-tert-butyl-3-ethylcyclohexane.
Problem 30b
Draw the most stable conformation of
b. trans-1-tert-butyl-2-methylcyclohexane.
Problem 30c
Draw the most stable conformation of
c. trans-1-tert-butyl-3-(1,1-dimethylpropyl)cyclohexane.
Problem 31a,b
Name the following compounds.
(a)
(b)
Problem 31c,d
Name the following compounds.
(c)
(d)
Problem 32
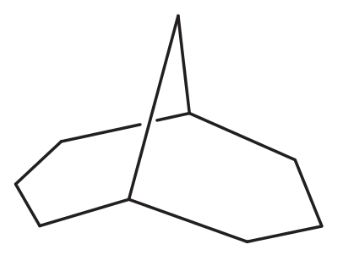
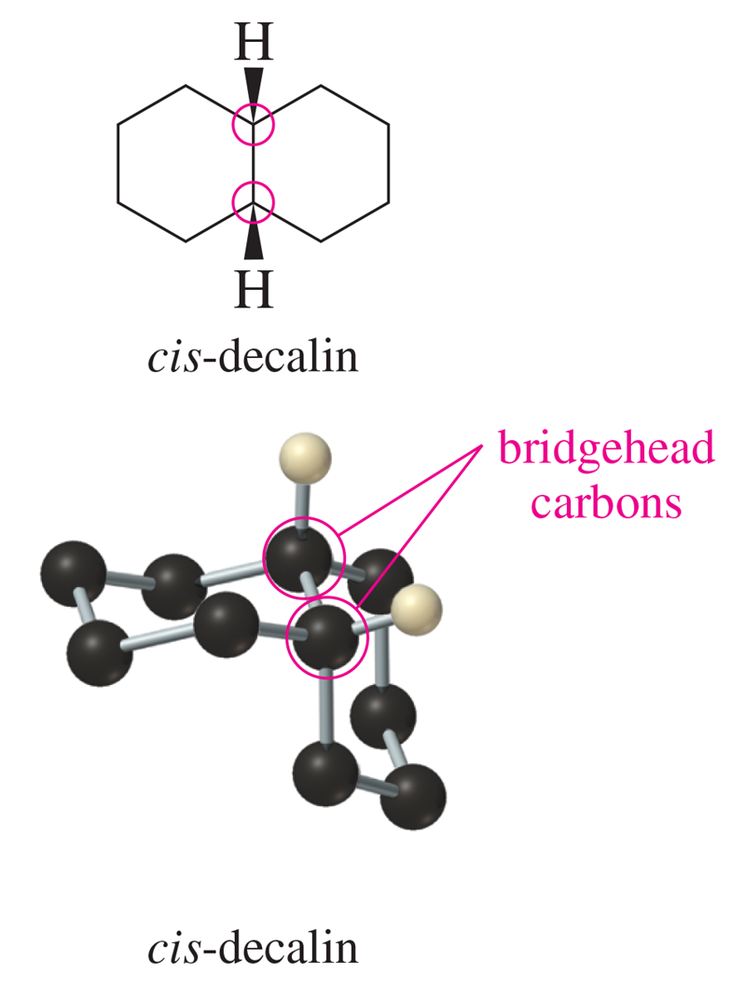
Use your models to do a chair–chair interconversion on each ring of the conformation of cis-decalin shown in Figure 3-27. Draw the conformation that results.
Problem 33a
a. There are 18 isomeric alkanes of molecular formula C8H18. Draw and name any eight of them.
Problem 33b
Draw and name the six isomeric cyclopentanes of molecular formula C7H14. These will include four constitutional isomers, of which two show geometric (cis-trans) stereoisomerism.
Problem 34a
Which of the following structures represent the same compound? Which ones represent different compounds?
(a)
Problem 34b
Which of the following structures represent the same compound? Which ones represent different compounds?
(b)