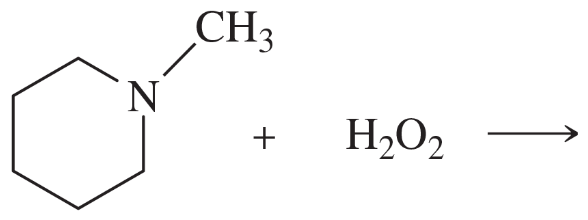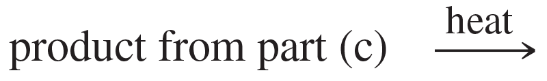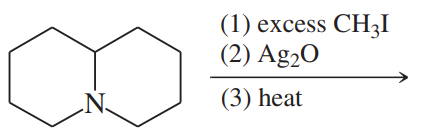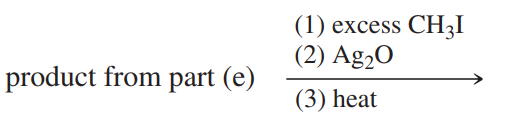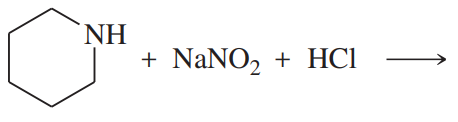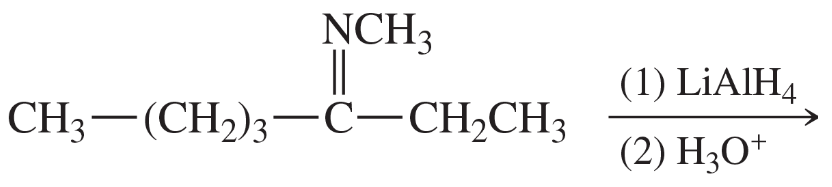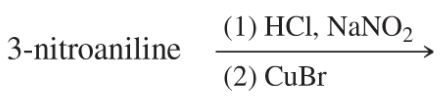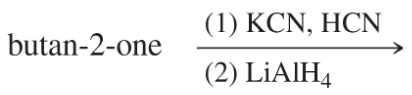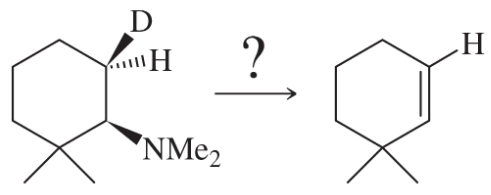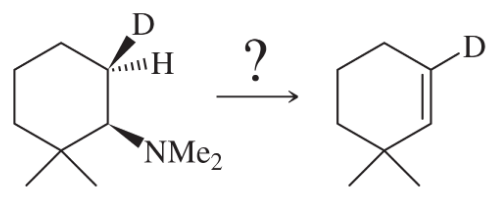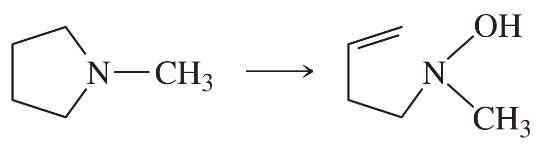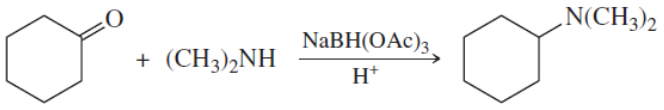Back
BackProblem 38c,d
Predict the products of the following reactions:
(c)
(d)
Problem 38e,f
Predict the products of the following reactions:
(e)
(f)
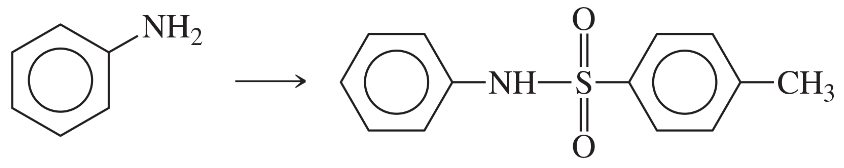
Problem 38g
Predict the products of the following reactions:
(g)
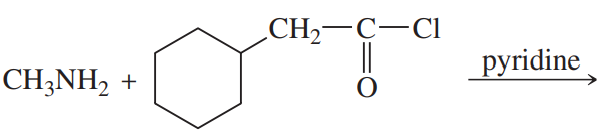
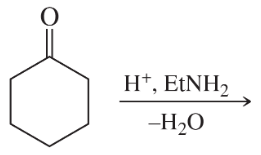
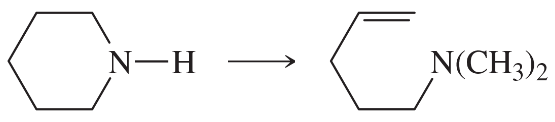
Problem 38i
Predict the products of the following reactions:
(i)
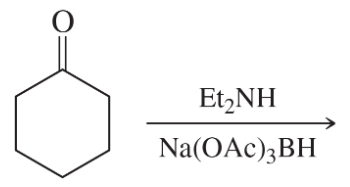
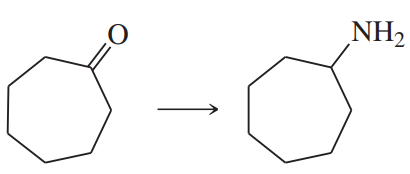
Problem 38k
Predict the products of the following reactions:
(k)
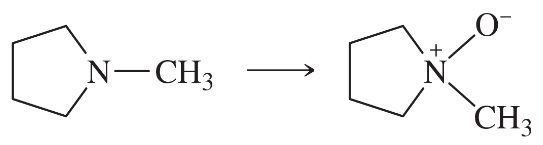
Problem 39c
Predict the products of the following reactions:
(c)
Problem 39d
Predict the products of the following reactions:
(d)
Problem 39g
Predict the products of the following reactions:
(g)
Problem 39h
Predict the products of the following reactions:
(h)
Problem 39i
Predict the products of the following reactions:
(i)
Problem 39j
Predict the products of the following reactions:
(j)
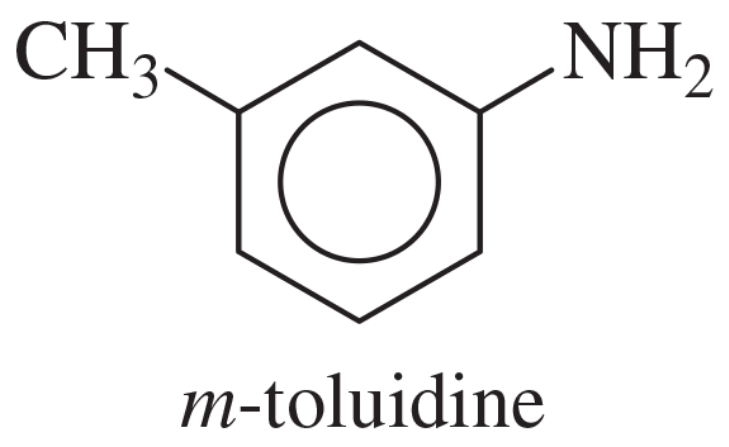
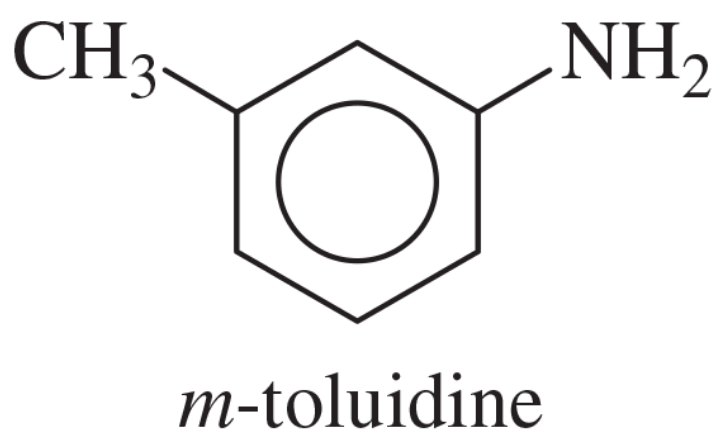
Problem 40a
Show how m-toluidine can be converted to the following compounds, using any necessary reagents.
(a)
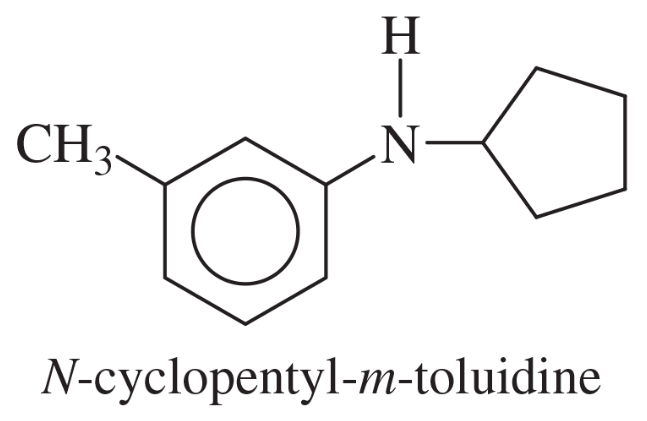
Problem 40b
Show how m-toluidine can be converted to the following compounds, using any necessary reagents.
(b)
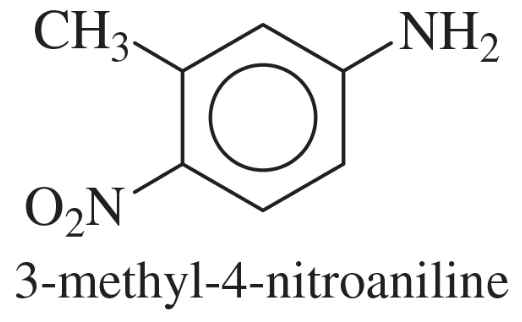
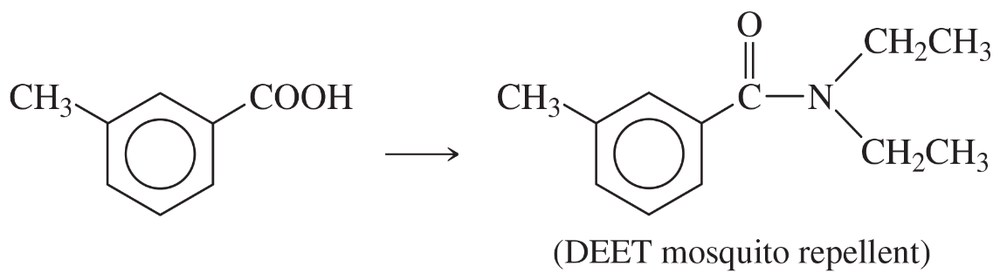
Problem 40e
Show how m-toluidine can be converted to the following compounds, using any necessary reagents.
(e)
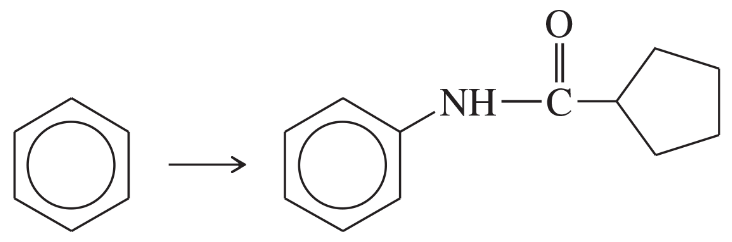
Problem 40f
Show how m-toluidine can be converted to the following compounds, using any necessary reagents.
(f)
Problem 41
The mass spectrum of tert-butylamine follows shows an intense base peak at m/z 58, and very little else. Use a diagram to show the cleavage that accounts for the base peak. Suggest why no molecular ion is visible in this spectrum.
Problem 42a
Using any necessary reagents, show how you would accomplish the following syntheses.
(a)
Problem 42b
Using any necessary reagents, show how you would accomplish the following syntheses.
(b)
Problem 42c
Using any necessary reagents, show how you would accomplish the following syntheses.
(c)
Problem 42d
Using any necessary reagents, show how you would accomplish the following syntheses.
(d)
Problem 42e,f
Using any necessary reagents, show how you would accomplish the following syntheses.
(e)
(f)
Problem 42g
Using any necessary reagents, show how you would accomplish the following syntheses.
(g)
Problem 43a
The following drugs are synthesized using the methods in this chapter and in previous chapters. Devise a synthesis for each, starting with any compounds containing no more than six carbon atoms.
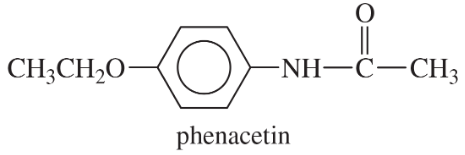
(a) Phenacetin, used with aspirin and caffeine in pain-relief medications.
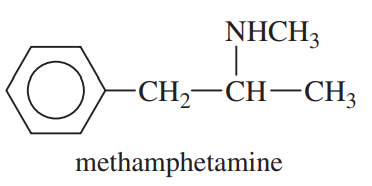
Problem 43b
The following drugs are synthesized using the methods in this chapter and in previous chapters. Devise a synthesis for each, starting with any compounds containing no more than six carbon atoms.
(b) Methamphetamine, once considered a safe diet pill, but now known to be addictive and destructive to brain tissue.
Problem 43c
The following drugs are synthesized using the methods in this chapter and in previous chapters. Devise a synthesis for each, starting with any compounds containing no more than six carbon atoms.
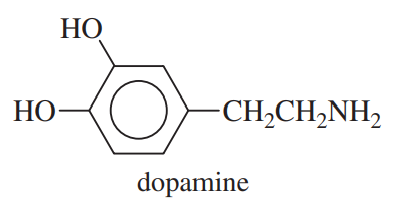
(c) Dopamine, one of the neurotransmitters in the brain. Parkinson’s disease is thought to result from a dopamine deficiency.
Problem 44
Synthesize Novocaine from benzene and any other reagents of four carbons or fewer.
Problem 45a
Synthesize from benzene. (Hint: All of these require diazonium ions.)
(a) 3-ethylbenzoic acid
Problem 45c
Synthesize from benzene. (Hint: All of these require diazonium ions.)
(c) 2-methyl-5-hydroxybenzoic acid
Problem 45d
Synthesize from benzene. (Hint: All of these require diazonium ions.)
(d) 4-methoxyaniline
Problem 46a
Propose mechanisms for the following reactions.
(a)