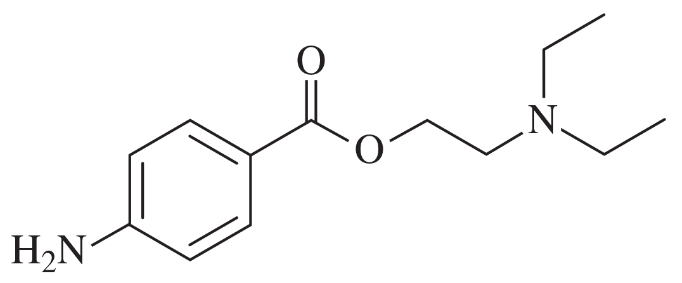
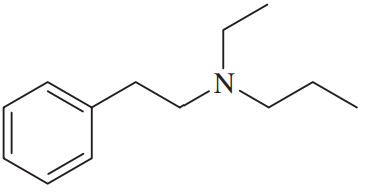
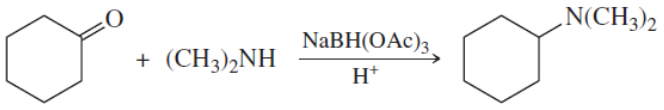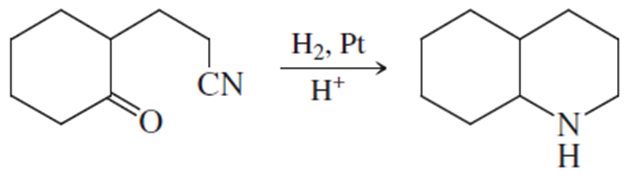Back
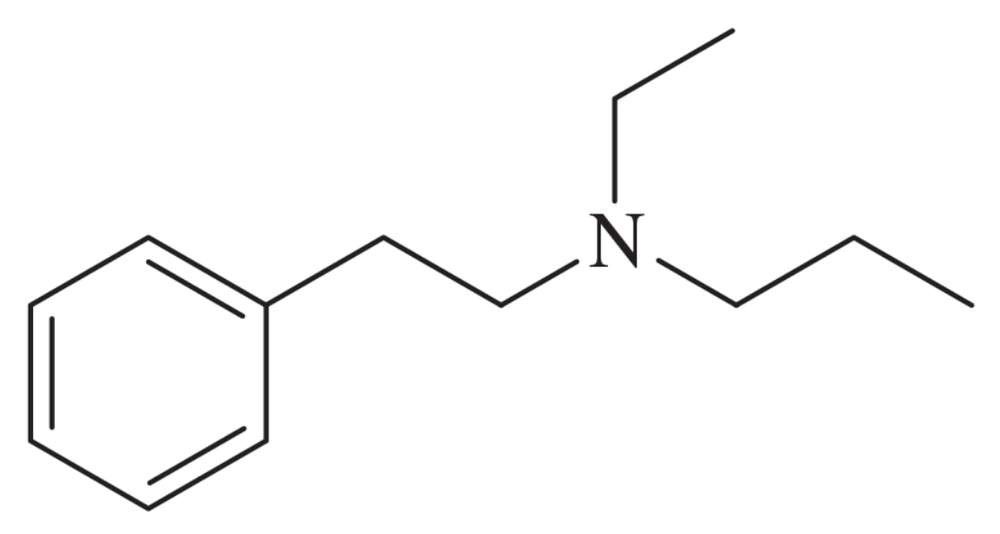
BackProblem 42g
Using any necessary reagents, show how you would accomplish the following syntheses.
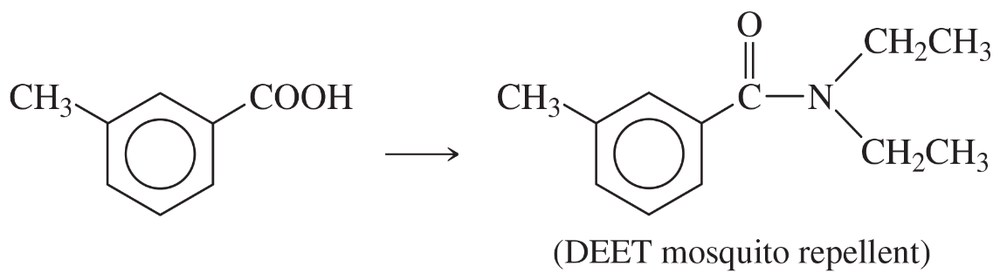
(g)
Problem 43a
The following drugs are synthesized using the methods in this chapter and in previous chapters. Devise a synthesis for each, starting with any compounds containing no more than six carbon atoms.
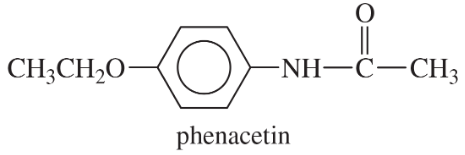
(a) Phenacetin, used with aspirin and caffeine in pain-relief medications.
Problem 43b
The following drugs are synthesized using the methods in this chapter and in previous chapters. Devise a synthesis for each, starting with any compounds containing no more than six carbon atoms.
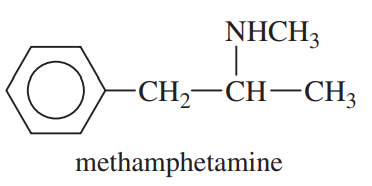
(b) Methamphetamine, once considered a safe diet pill, but now known to be addictive and destructive to brain tissue.
Problem 43c
The following drugs are synthesized using the methods in this chapter and in previous chapters. Devise a synthesis for each, starting with any compounds containing no more than six carbon atoms.
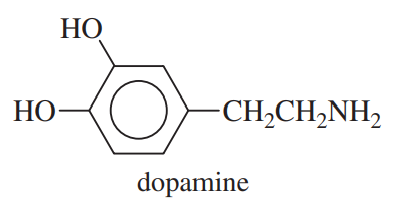
(c) Dopamine, one of the neurotransmitters in the brain. Parkinson’s disease is thought to result from a dopamine deficiency.
Problem 44
Synthesize Novocaine from benzene and any other reagents of four carbons or fewer.
Problem 45a
Synthesize from benzene. (Hint: All of these require diazonium ions.)
(a) 3-ethylbenzoic acid
Problem 45c
Synthesize from benzene. (Hint: All of these require diazonium ions.)
(c) 2-methyl-5-hydroxybenzoic acid
Problem 45d
Synthesize from benzene. (Hint: All of these require diazonium ions.)
(d) 4-methoxyaniline
Problem 46a
Propose mechanisms for the following reactions.
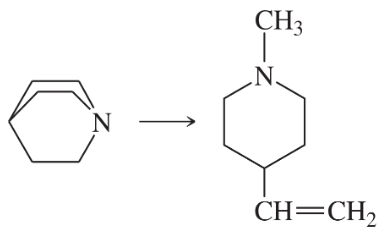
(a)
Problem 46b
Propose mechanisms for the following reactions.
(b)
Problem 47a
The two most general amine syntheses are the reductive amination of carbonyl compounds and the reduction of amides. Show how these techniques can be used to accomplish the following syntheses.
(a) benzoic acid → benzylamine
Problem 47b
The two most general amine syntheses are the reductive amination of carbonyl compounds and the reduction of amides. Show how these techniques can be used to accomplish the following syntheses.
(b) benzaldehyde → benzylamine
Problem 47c
The two most general amine syntheses are the reductive amination of carbonyl compounds and the reduction of amides. Show how these techniques can be used to accomplish the following syntheses.
(c) pyrrolidine → N-ethylpyrrolidine
Problem 47d
The two most general amine syntheses are the reductive amination of carbonyl compounds and the reduction of amides. Show how these techniques can be used to accomplish the following syntheses.
(d) cyclohexanone → N-cyclohexylpyrrolidine
Problem 48a
Several additional amine syntheses are effectively limited to making primary amines. The reduction of azides and nitro compounds and the Gabriel synthesis leave the carbon chain unchanged. Formation and reduction of a nitrile adds one carbon atom. Show how these amine syntheses can be used for the following conversions.
(a) allyl bromide → allylamine
Problem 48c
Several additional amine syntheses are effectively limited to making primary amines. The reduction of azides and nitro compounds and the Gabriel synthesis leave the carbon chain unchanged. Formation and reduction of a nitrile adds one carbon atom. Show how these amine syntheses can be used for the following conversions.
(c) 1-bromo-3-phenylheptane → 3-phenylheptan-1-amine
Problem 49a
Show how you can synthesize the following tertiary amine three different ways, each using a different secondary amine and adding the final substituent by
(a) reductive amination (3 ways).
Problem 49b
Show how you can synthesize the following tertiary amine three different ways, each using a different secondary amine and adding the final substituent by (b) acylation–reduction (3 ways).
Problem 50a
Show how you can synthesize the following compounds starting with benzene, toluene, and alcohols containing no more than four carbon atoms as your organic starting materials. Assume that para is the major product (and separable from ortho) in ortho, para mixtures.
(a) pentan-1-amine
Problem 50b
Show how you can synthesize the following compounds starting with benzene, toluene, and alcohols containing no more than four carbon atoms as your organic starting materials. Assume that para is the major product (and separable from ortho) in ortho, para mixtures.
(b) N-methylbutan-1-amine
Problem 50c
Show how you can synthesize the following compounds starting with benzene, toluene, and alcohols containing no more than four carbon atoms as your organic starting materials. Assume that para is the major product (and separable from ortho) in ortho, para mixtures.
(c) N-ethyl-N-propylbutan-2-amine
Problem 50d
Show how you can synthesize the following compounds starting with benzene, toluene, and alcohols containing no more than four carbon atoms as your organic starting materials. Assume that para is the major product (and separable from ortho) in ortho, para mixtures.
(d) N-benzylpropan-1-amine
Problem 50g
Show how you can synthesize the following compounds starting with benzene, toluene, and alcohols containing no more than four carbon atoms as your organic starting materials. Assume that para is the major product (and separable from ortho) in ortho, para mixtures.
(g) 4-isobutylaniline
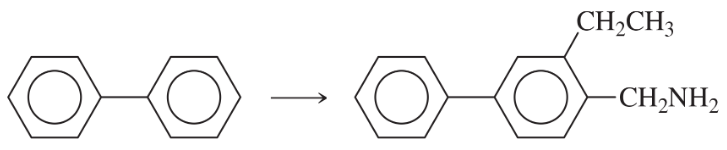
Problem 51b
Using any necessary reagents, show how you can accomplish the following multistep syntheses.
(b)
Problem 51c
Using any necessary reagents, show how you can accomplish the following multistep syntheses.
(c)
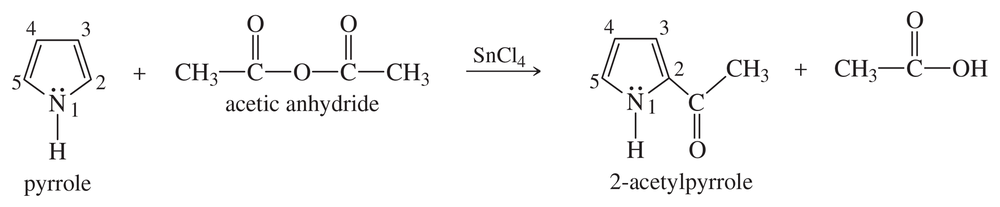
Problem 54a
Pyrrole undergoes electrophilic aromatic substitution more readily than benzene, and mild reagents and conditions are sufficient. These reactions normally occur at the 2-position rather than the 3-position, as shown in the following example.
a. Propose a mechanism for the acetylation of pyrrole just shown. You may begin with pyrrole and the acylium ion, CH3C≡O+. Be careful to draw all the resonance structures of the intermediate.
Problem 54b
Pyrrole undergoes electrophilic aromatic substitution more readily than benzene, and mild reagents and conditions are sufficient. These reactions normally occur at the 2-position rather than the 3-position, as shown in the following example.
b. Explain why pyrrole reacts more readily than benzene, and also why substitution occurs primarily at the 2-position rather than the 3-position.
Problem 56
The following spectra for A and B correspond to two structural isomers. The NMR singlet at δ1.16 in spectrum A disappears when the sample is shaken with D2O. The singlet at δ0.6 ppm in the spectrum of B disappears on shaking with D2O. Propose structures for these isomers, and show how your structures correspond to the spectra. Show what cleavage is responsible for the base peak at m/z 44 in the mass spectrum of A and the prominent peak at m/z 58 in the mass spectrum of B.
<IMAGE>
Problem 60a
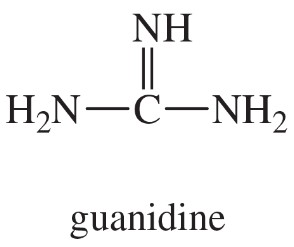
Guanidine (shown) is about as strong a base as hydroxide ion. Explain why guanidine is a much stronger base than most other amines.
Problem 60b
Show why p-nitroaniline is a much weaker base (3 pKb units weaker) than aniline.