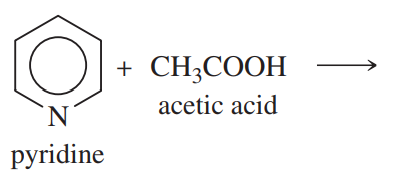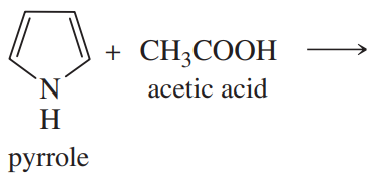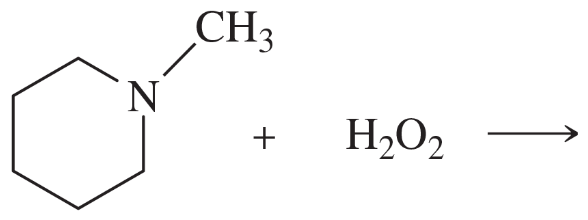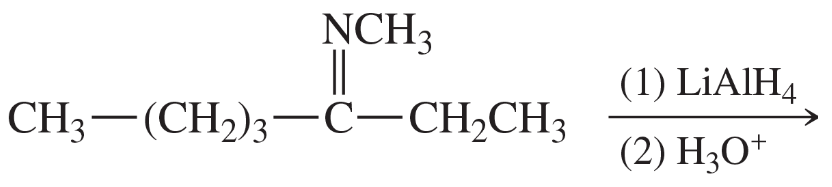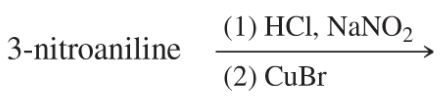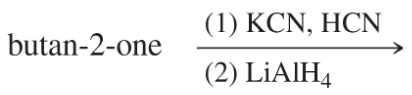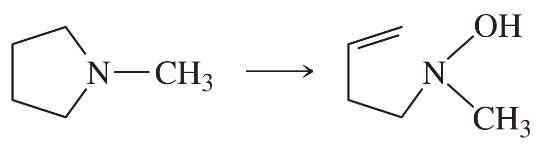Back
BackProblem 34d,e
Within each structure, rank the indicated nitrogens by increasing basicity.
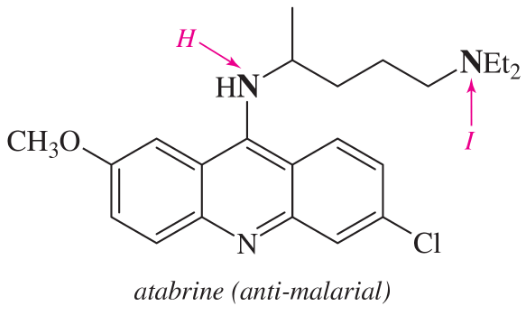
(d)
(e)
Problem 34f
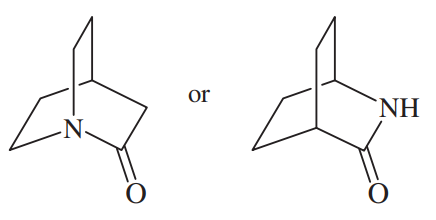
Within each structure, rank the indicated nitrogens by increasing basicity.
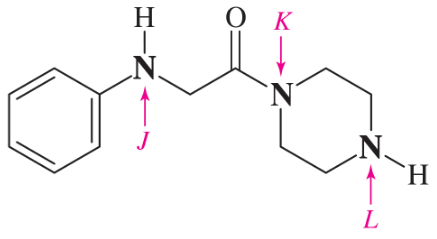
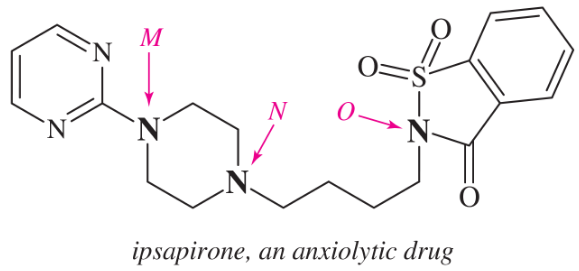
(f)
Problem 35a,b
In each pair of compounds, select the stronger base, and explain your choice.
(a) HOCH2CH2NH2 or CH3CH2NH2
(b) PhNH2 or PhCH2NH2
Problem 35c,d
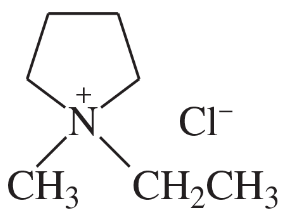
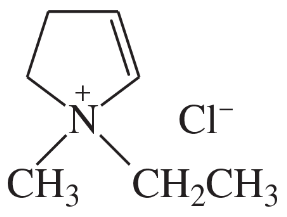
In each pair of compounds, select the stronger base, and explain your choice.
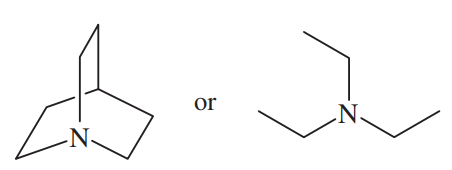
(c)
(d)
Problem 36a,b,c
Which of the following compounds are capable of being resolved into enantiomers?
(a) N-ethyl-N-methylaniline
(b) 2-methylpiperidine
(c) 1-methylpiperidine
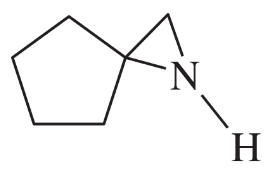
Problem 36d,e,f
Which of the following compounds are capable of being resolved into enantiomers?
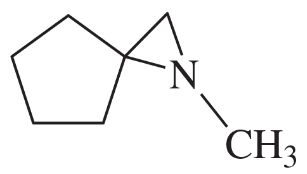
(d) 1,2,2-trimethylaziridine
(e)
(f)
Problem 36g,h
Which of the following compounds are capable of being resolved into enantiomers?
(g)
(h)
Problem 37a,b
Complete the following proposed acid–base reactions, and predict whether the reactants or products are favored.
(a)
(b)
Problem 38a
Predict the products of the following reactions:
(a) excess NH3 + Ph–CH2CH2CH2Br →
Problem 38c,d
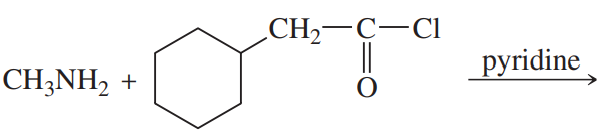
Predict the products of the following reactions:
(c)
(d)
Problem 38e,f
Predict the products of the following reactions:
(e)
(f)
Problem 38g
Predict the products of the following reactions:
(g)
Problem 38i
Predict the products of the following reactions:
(i)
Problem 38k
Predict the products of the following reactions:
(k)
Problem 39c
Predict the products of the following reactions:
(c)
Problem 39d
Predict the products of the following reactions:
(d)
Problem 39g
Predict the products of the following reactions:
(g)
Problem 39h
Predict the products of the following reactions:
(h)
Problem 39i
Predict the products of the following reactions:
(i)
Problem 39j
Predict the products of the following reactions:
(j)
Problem 40a
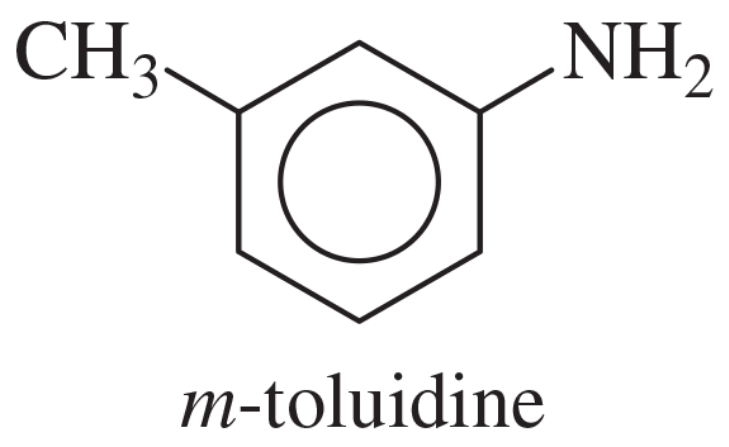
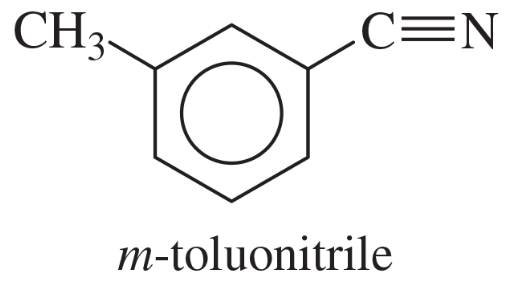
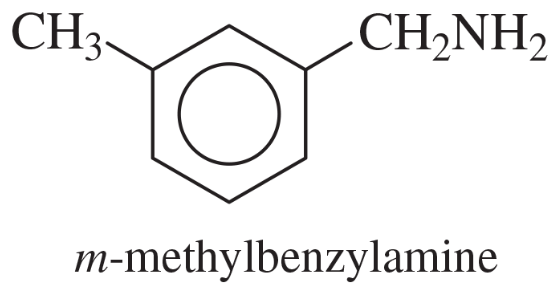
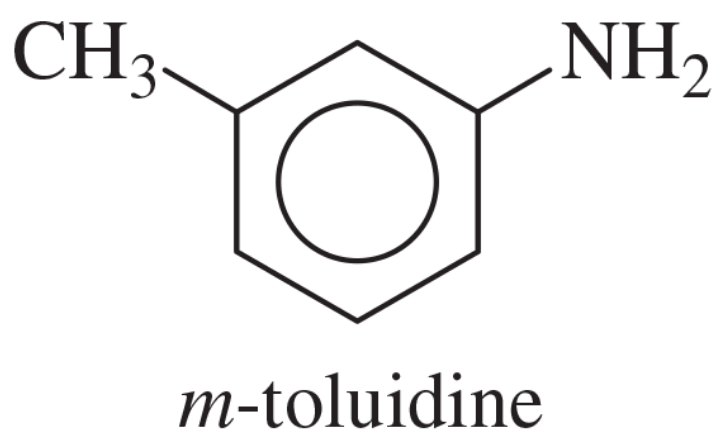
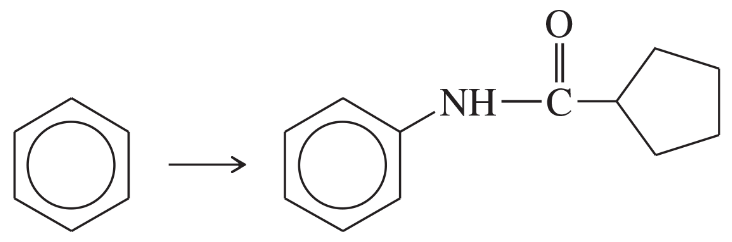
Show how m-toluidine can be converted to the following compounds, using any necessary reagents.
(a)
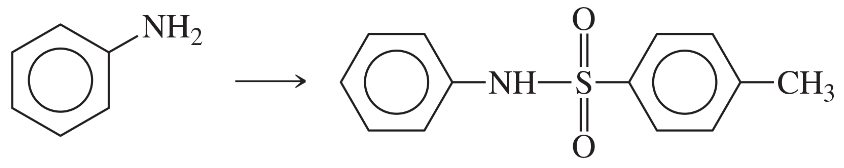
Problem 40b
Show how m-toluidine can be converted to the following compounds, using any necessary reagents.
(b)
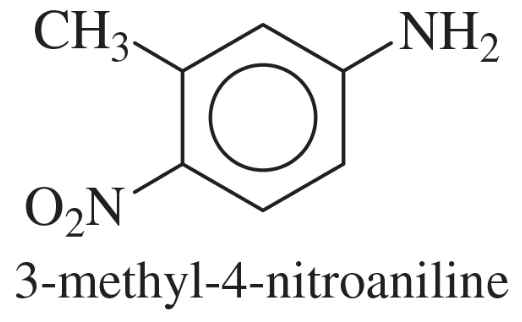
Problem 40e
Show how m-toluidine can be converted to the following compounds, using any necessary reagents.
(e)
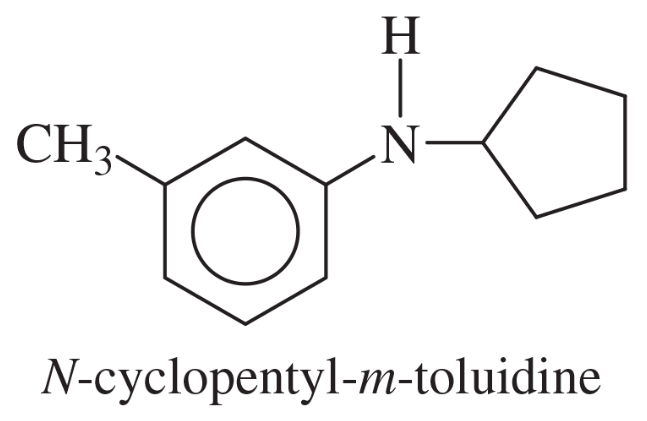
Problem 40f
Show how m-toluidine can be converted to the following compounds, using any necessary reagents.
(f)
Problem 41
The mass spectrum of tert-butylamine follows shows an intense base peak at m/z 58, and very little else. Use a diagram to show the cleavage that accounts for the base peak. Suggest why no molecular ion is visible in this spectrum.
Problem 42a
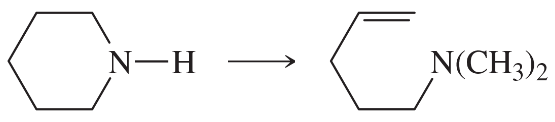
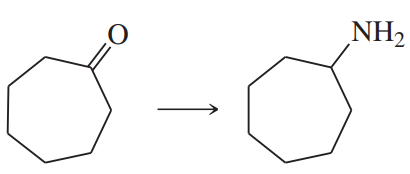
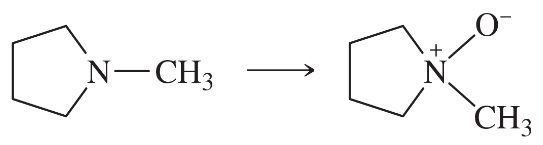
Using any necessary reagents, show how you would accomplish the following syntheses.
(a)
Problem 42b
Using any necessary reagents, show how you would accomplish the following syntheses.
(b)
Problem 42c
Using any necessary reagents, show how you would accomplish the following syntheses.
(c)
Problem 42d
Using any necessary reagents, show how you would accomplish the following syntheses.
(d)
Problem 42e,f
Using any necessary reagents, show how you would accomplish the following syntheses.
(e)
(f)