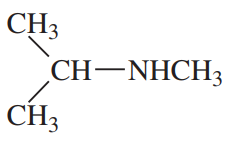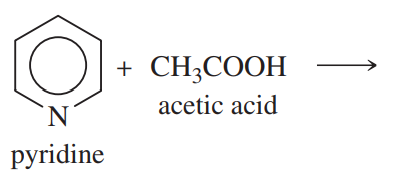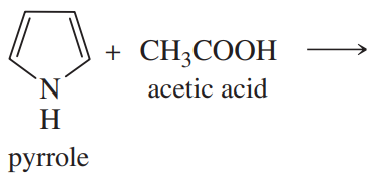Back
BackProblem 26a,b,c
Show how to synthesize the following amines from the indicated starting materials by reductive amination.
(a) benzylmethylamine from benzaldehyde
(b) N-benzylpiperidine from piperidine
(c) N-cyclohexylaniline from cyclohexanone
Problem 26e
Show how to synthesize the following amines from the indicated starting materials by reductive amination.
(e)
Problem 27
Show how to synthesize the following amines from the indicated starting materials by acylation–reduction.
(a) N-butylpiperidine from piperidine
(b) N-benzylaniline from aniline
Problem 28
Addition of one equivalent of ammonia to 1-bromoheptane gives a mixture of heptan-1-amine, some dialkylamine, some trialkylamine, and even some tetraalkylammonium bromide.
(a) Give a mechanism to show how this reaction takes place, as far as the dialkylamine.
(b) How would you modify the procedure to get an acceptable yield of heptan-1-amine?
Problem 29a,b,c
Show how Gabriel syntheses are used to prepare the following amines.
(a) benzylamine
(b) hexan-1-amine
(c) γ-aminobutyric acid
Problem 30a
Show how you would accomplish the following synthetic conversions.
(a) benzyl bromide → benzylamine
Problem 30b
Show how you would accomplish the following synthetic conversions.
(b) 1-bromo-2-phenylethane → 3-phenylpropan-1-amine
Problem 30c
Show how you would accomplish the following synthetic conversions.
(c) pentanoic acid → pentan-1-amine
Problem 30d
Show how you would accomplish the following synthetic conversions.
(d) pentanoic acid → hexan-1-amine
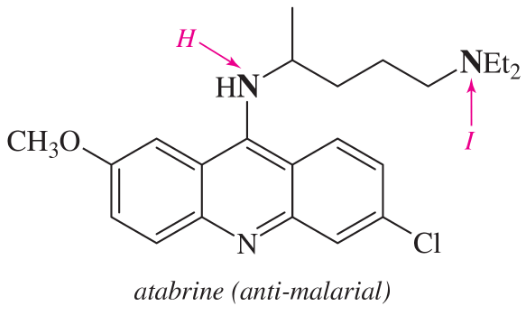
Problem 30e
Show how you would accomplish the following synthetic conversions.
(e) (R)-2-bromobutane → (S)-butan-2-amine
Problem 30f
Show how you would accomplish the following synthetic conversions.
(f) (R)-2-bromobutane → (S)-2-methylbutan-1-amine
Problem 31a
Show how to prepare the following aromatic amines by aromatic nitration, followed by reduction. You may use benzene and toluene as your aromatic starting materials.
(a) aniline
Problem 31d
Show how to prepare the following aromatic amines by aromatic nitration, followed by reduction. You may use benzene and toluene as your aromatic starting materials.
(d) m-aminobenzoic acid
Problem 32a,b,c
For each compound,
(1) classify the nitrogen-containing functional groups.
(2) provide an acceptable name.
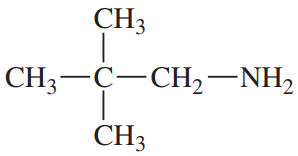
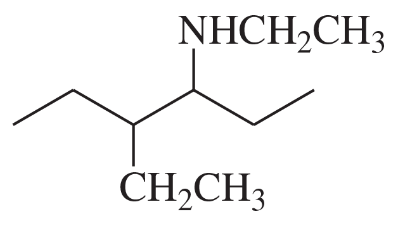
(a)
(b)
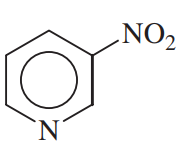
(c)
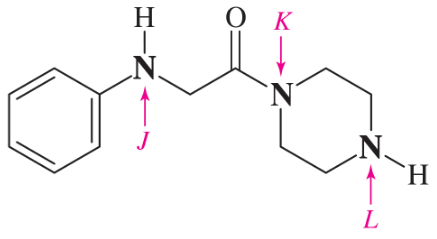
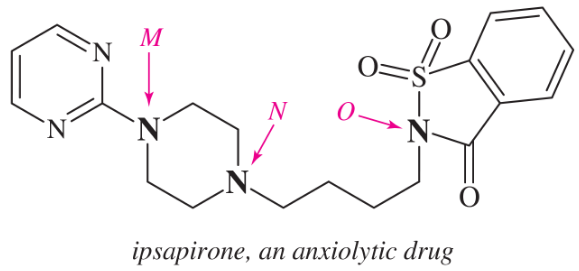
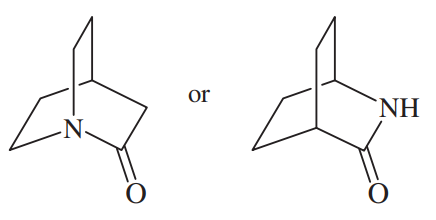
Problem 32g,h
For each compound,
(1) classify the nitrogen-containing functional groups.
(2) provide an acceptable name.
(g)
(h)
Problem 33a
Rank the amines in each set in order of increasing basicity.
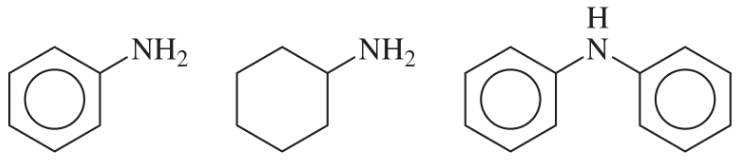
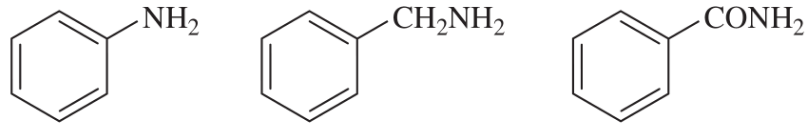
(a)
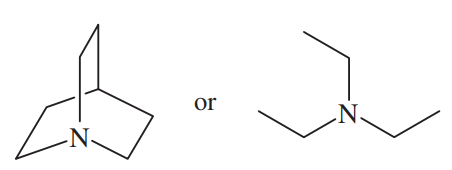
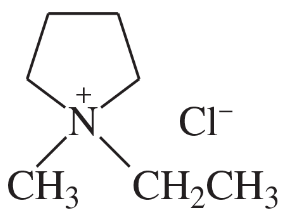
Problem 33c
Rank the amines in each set in order of increasing basicity.
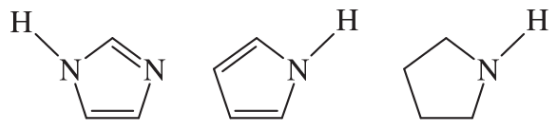
(c)
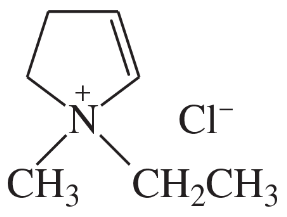
Problem 33d
Rank the amines in each set in order of increasing basicity.
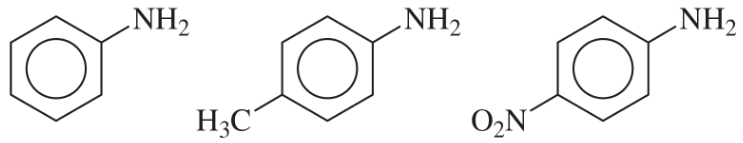
(d)
Problem 33e
Rank the amines in each set in order of increasing basicity.
(e)
Problem 34a,b
Within each structure, rank the indicated nitrogens by increasing basicity.
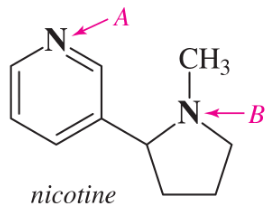
(a)
(b)
Problem 34c
Within each structure, rank the indicated nitrogens by increasing basicity.
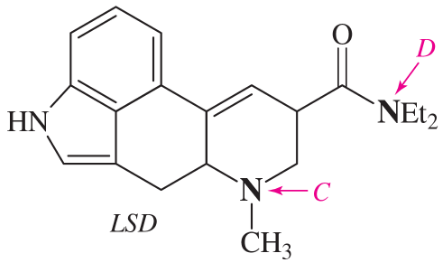
(c)
Problem 34d,e
Within each structure, rank the indicated nitrogens by increasing basicity.
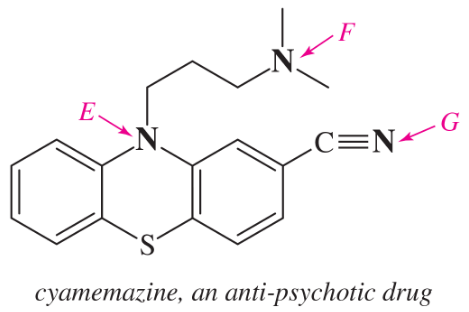
(d)
(e)
Problem 34f
Within each structure, rank the indicated nitrogens by increasing basicity.
(f)
Problem 35a,b
In each pair of compounds, select the stronger base, and explain your choice.
(a) HOCH2CH2NH2 or CH3CH2NH2
(b) PhNH2 or PhCH2NH2
Problem 35c,d
In each pair of compounds, select the stronger base, and explain your choice.
(c)
(d)
Problem 36a,b,c
Which of the following compounds are capable of being resolved into enantiomers?
(a) N-ethyl-N-methylaniline
(b) 2-methylpiperidine
(c) 1-methylpiperidine
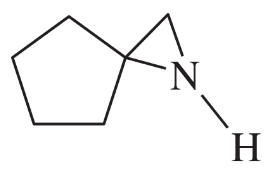
Problem 36d,e,f
Which of the following compounds are capable of being resolved into enantiomers?
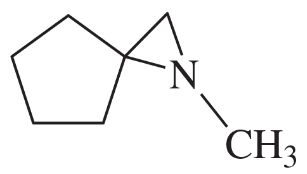
(d) 1,2,2-trimethylaziridine
(e)
(f)
Problem 36g,h
Which of the following compounds are capable of being resolved into enantiomers?
(g)
(h)
Problem 37a,b
Complete the following proposed acid–base reactions, and predict whether the reactants or products are favored.
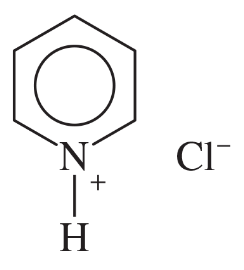
(a)
(b)
Problem 38a
Predict the products of the following reactions:
(a) excess NH3 + Ph–CH2CH2CH2Br →