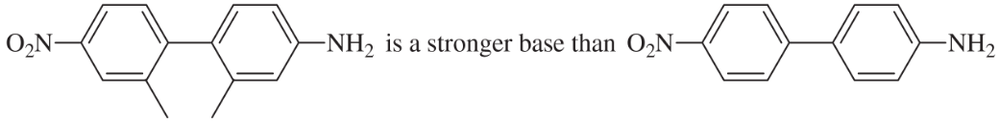Back
BackProblem 60c
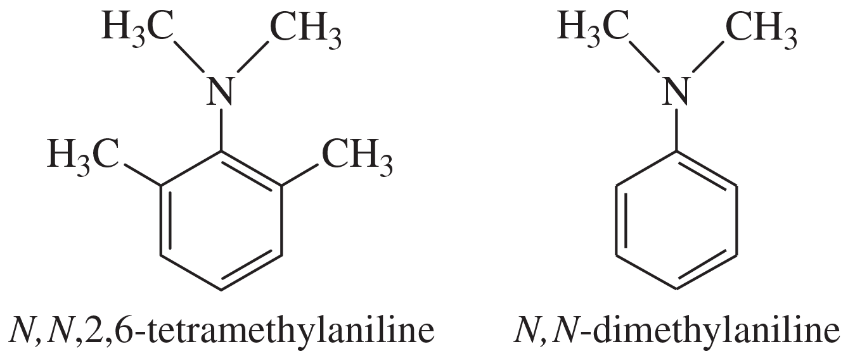
Explain why N,N,2,6-tetramethylaniline (shown) is a much stronger base than N,N-dimethylaniline.
Problem 61a
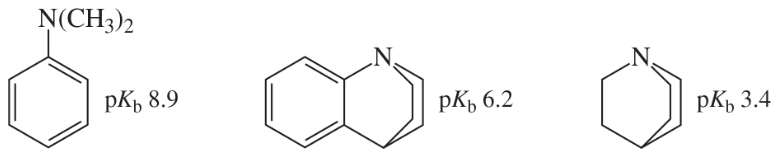
Basicity depends on availability of an electron pair to bond a proton. Correlate structural effects in these amines with their basicities.
(a) Explain this order:
Problem 61b
Basicity depends on availability of an electron pair to bond a proton. Correlate structural effects in these amines with their basicities.
(b) Explain:
Problem 61c
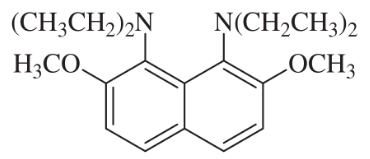
Basicity depends on availability of an electron pair to bond a proton. Correlate structural effects in these amines with their basicities.
(c) The pKb of this compound is −2.3, making it not only a stronger base than a typical aniline, but even stronger than hydroxide ion. Explain its remarkable basicity.
Problem 63c,d
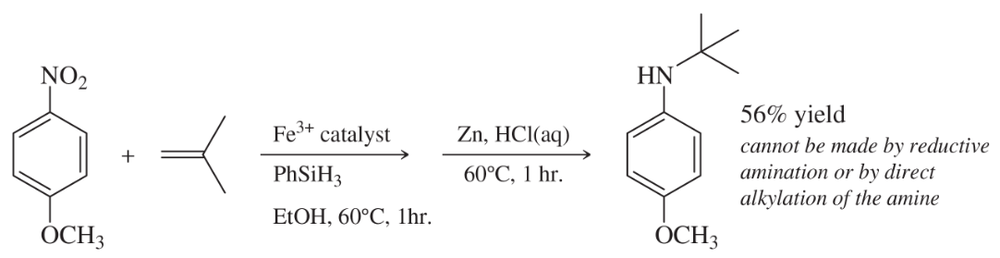
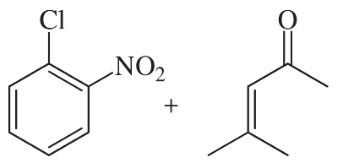
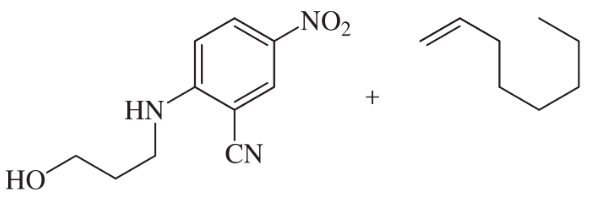
Reductive amination of aldehydes and ketones is a versatile method for attaching alkyl groups to amines, but the alkyl group is restricted to a 1° or 2° carbon by this method. Prof. Phil Baran of Scripps Research Institute has reported (Science, 2015, 348 (6237), 886–891) a novel way to reduce an aromatic nitro group and add the resulting amine to an alkene so that the aromatic amine is bonded to a 3° carbon—all in a continuous sequence of reactions.
For example:
Predict the products using these starting materials, all of which are reported in this paper.
(c)
(d)
Problem 64a
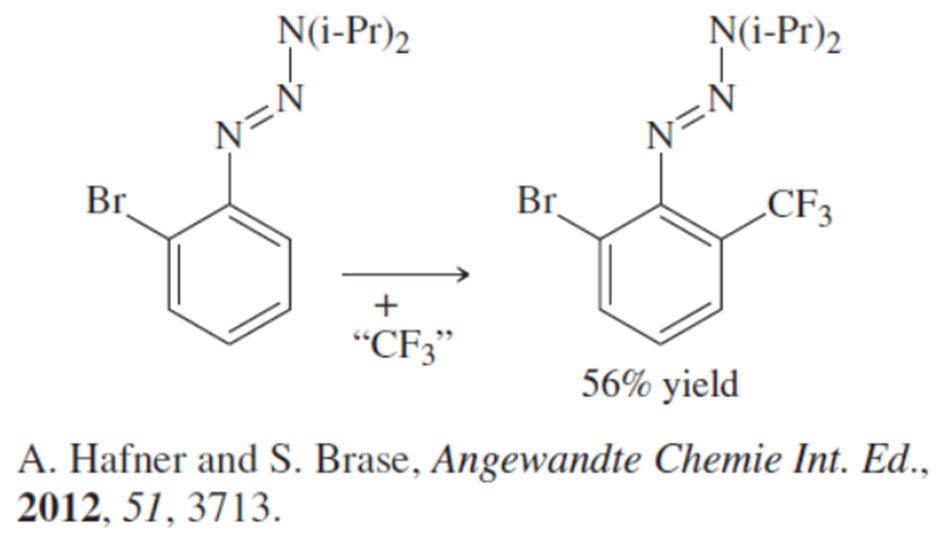
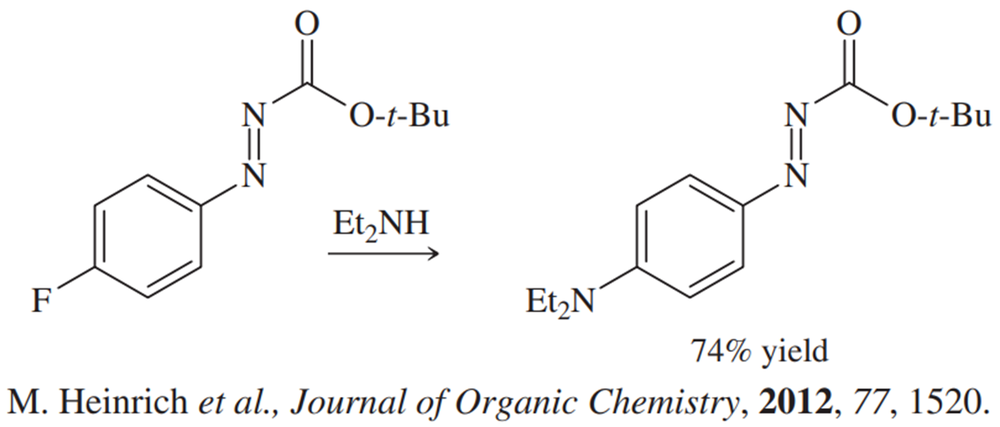
Show how the substituents containing the azo group (N=N) can facilitate both electrophilic and nucleophilic aromatic substitution.
(a)
Problem 64b
Show how the substituents containing the azo group (N=N) can facilitate both electrophilic and nucleophilic aromatic substitution.
(b)
Problem 65a,b
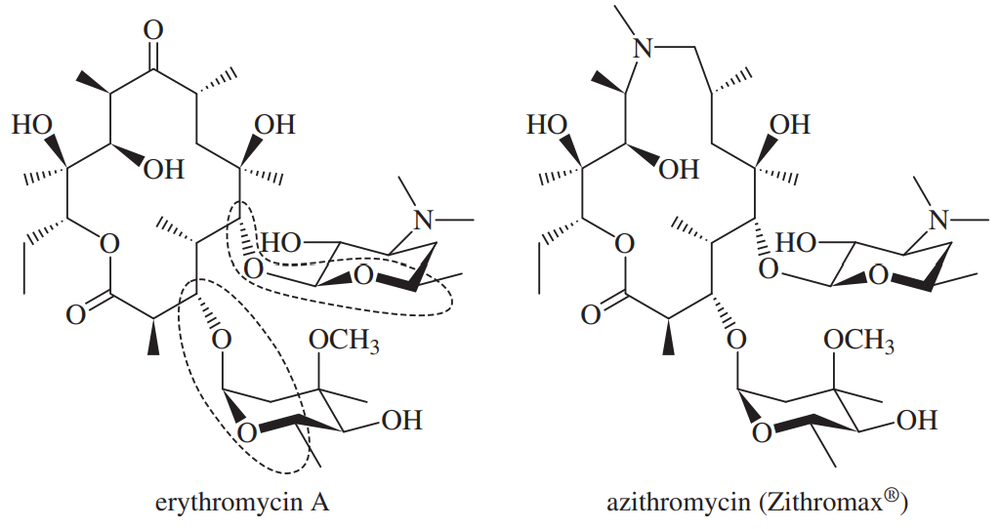
Macrolide antibiotics all have large rings (macrocycle) in which an ester makes the ring; a cyclic ester is termed a lactone. One example is erythromycin A, first isolated from soil bacteria in the 1950s. Over time, some pathogenic bacteria have developed resistance to erythromycin by evolving an enzymatic mechanism to cleave the macrocycle at the ketone. To counter this resistance, chemists modified the erythromycin structure to replace the ketone with an amine that the bacteria could not detoxify. This modified antibiotic, azithromycin, trade name Zithromax®, is one of the most prescribed drugs in the world for respiratory infections.
(a) Identify the lactone group in each structure that merits the classification as macrolides.
(b) Two groups are circled. What type of functional group are they? Explain.
Problem 65c,d
Macrolide antibiotics all have large rings (macrocycle) in which an ester makes the ring; a cyclic ester is termed a lactone. One example is erythromycin A, first isolated from soil bacteria in the 1950s. Over time, some pathogenic bacteria have developed resistance to erythromycin by evolving an enzymatic mechanism to cleave the macrocycle at the ketone. To counter this resistance, chemists modified the erythromycin structure to replace the ketone with an amine that the bacteria could not detoxify. This modified antibiotic, azithromycin, trade name Zithromax®, is one of the most prescribed drugs in the world for respiratory infections.
(c) Identify the ketone in erythromycin targeted by bacteria as the site for detoxification.
(d) Identify the amine in azithromycin. What type of amine is it?