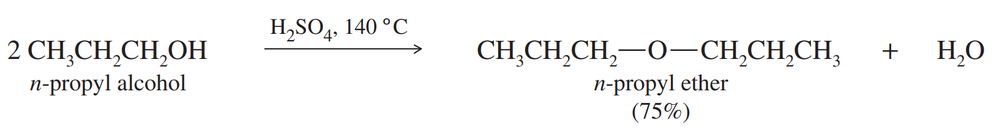Back
BackProblem 10f
Show how the following ethers might be synthesized using (1) alkoxymercuration–demercuration and (2) the Williamson synthesis. (When one of these methods cannot be used for the given ether, point out why it will not work.)
f. tert-butyl phenyl ether
Problem 11a
Explain why bimolecular condensation is a poor method for making unsymmetrical ethers such as ethyl methyl ether.
Problem 12
Propose a mechanism for the acid-catalyzed condensation of n-propyl alcohol to n-propyl ether, as shown above. When the temperature is allowed to rise too high, propene is formed. Propose a mechanism for the formation of propene, and explain why it is favored at higher temperatures.
Problem 13
Which of the following ethers can be formed in good yield by condensation of the corresponding alcohols? For those that cannot be formed by condensation, suggest an alternative method that will work.
(a) dibutyl ether
(b) ethyl n-propyl ether
(c) di-sec-butyl ether
Problem 14a
Propose a mechanism for the following reaction.
Problem 15a,b,c
Predict the products of the following reactions. An excess of acid is available in each case.
(a) ethoxycyclohexane + HBr
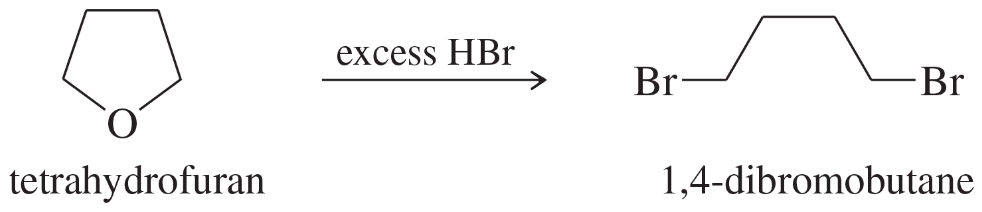
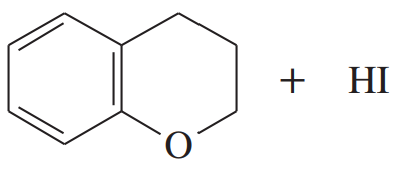
(b) tetrahydropyran + HI
(c) anisole (methoxybenzene) + HBr
Problem 15c
Predict the products of the following reactions. An excess of acid is available in each case.
(c) anisole (methoxybenzene) + HBr
Problem 15d
Predict the products of the following reactions. An excess of acid is available in each case.
(d)
Problem 16
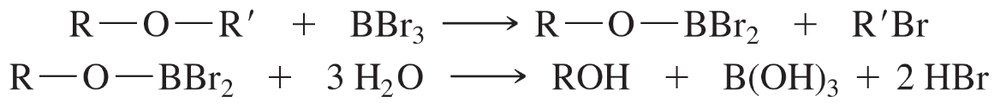
Boron tribromide (BBr3) cleaves ethers to give alkyl halides and alcohols.
The reaction is thought to involve attack by a bromide ion on the Lewis acid–base adduct of the ether with BBr3 (a strong Lewis acid). Propose a mechanism for the reaction of butyl methyl ether with BBr3 to give (after hydrolysis) butan-1-ol and bromomethane.
Problem 17
Show how you would synthesize butyl isopropyl sulfide using butan-1-ol, propan-2-ol, and any solvents and reagents you need.
Problem 18
Mustard gas, Cl–CH2CH2–S–CH2CH2–Cl, was used as a poisonous chemical agent in World War I. Mustard gas is much more toxic than a typical primary alkyl chloride. Its toxicity stems from its ability to alkylate amino groups on important metabolic enzymes, rendering the enzymes inactive.
a. Propose a mechanism to explain why mustard gas is an exceptionally potent alkylating agent.
b. Bleach (sodium hypochlorite, NaOCl, a strong oxidizing agent) neutralizes and inactivates mustard gas. Bleach is also effective on organic stains because it oxidizes colored compounds to colorless compounds. Propose products that might be formed by the reaction of mustard gas with bleach.
Problem 19
Show how you would use a protecting group to convert 4-bromobutan-1-ol to hept-5-yn-1-ol.
Problem 20a,b,c
Show how you would accomplish the following transformations. Some of these examples require more than one step.
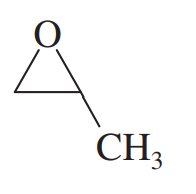
(a) 2-methylpropene → 2,2-dimethyloxirane
(b) 1-phenylethanol → 2-phenyloxirane
(c) 5-chloropent-1-ene → tetrahydropyran
Problem 20d
Show how you would accomplish the following transformations. Some of these examples require more than one step.
(d) 5-chloropent-1-ene → 2-methyltetrahydrofuran
Problem 20e
Show how you would accomplish the following transformations. Some of these examples require more than one step.
(e) 2-chlorohexan-1-ol → 1,2-epoxyhexane
Problem 21
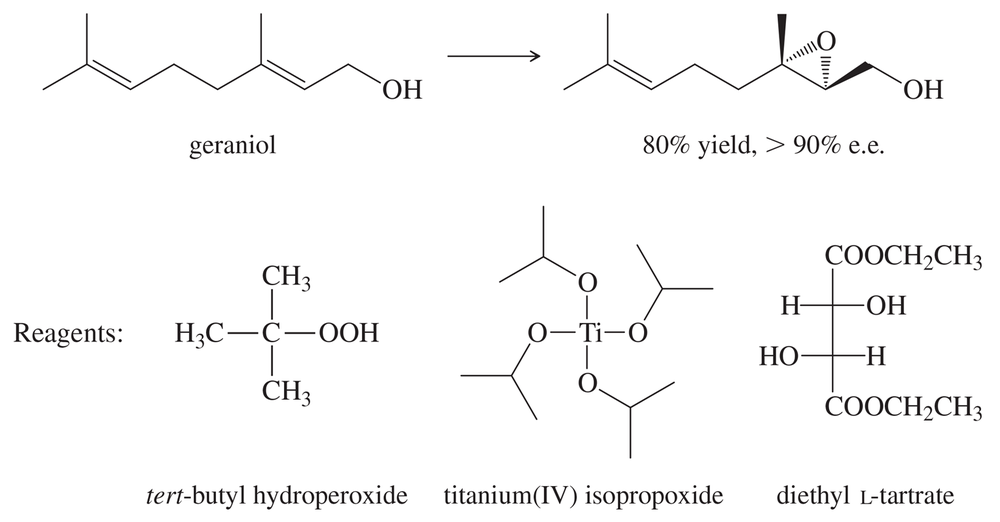
The 2001 Nobel Prize in Chemistry was awarded to three organic chemists who have developed methods for catalytic asymmetric syntheses. An asymmetric (or enantioselective) synthesis is one that converts an achiral starting material into mostly one enantiomer of a chiral product. K. Barry Sharpless (The Scripps Research Institute) developed an asymmetric epoxidation of allylic alcohols that gives excellent chemical yields and greater than 90% enantiomeric excess.
The Sharpless epoxidation uses tert-butyl hydroperoxide, titanium(IV) isopropoxide, and a dialkyl tartrate ester as the reagents. The following epoxidation of geraniol is typical.
(a) Which of these reagents is most likely to be the actual oxidizing agent? That is, which reagent is reduced in the reaction? What is the likely function of the other reagents?
(b) When achiral reagents react to give a chiral product, that product is normally formed as a racemic mixture of enantiomers. How can the Sharpless epoxidation give just one nearly pure enantiomer of the product?
(c) Draw the other enantiomer of the product. What reagents would you use if you wanted to epoxidize geraniol to give this other enantiomer?
Problem 22

Propose mechanisms for the epoxidation and ring-opening steps of the epoxidation and hydrolysis of trans-but-2-ene shown above. Predict the product of the same reaction with cis-but-2-ene.
Problem 23
Cellosolve® is the trade name for 2-ethoxyethanol, a common industrial solvent. This compound is produced in chemical plants that use ethylene as their only organic feedstock. Show how you would accomplish this industrial process.
Problem 24a
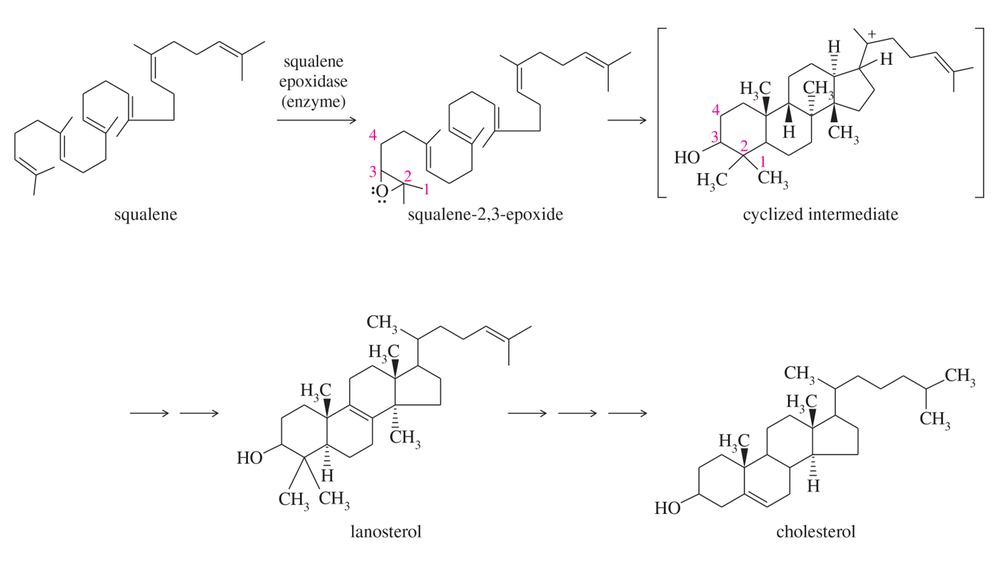
Show the rest of the mechanism for formation of the cyclized intermediate in Figure 14-6.
Problem 26a,b,c
Predict the major product when each reagent reacts with ethylene oxide.
(a) NaOCH2CH3 (sodium ethoxide)
(b) NaNH2 (sodium amide)
(c) NaSPh (sodium thiophenoxide)
Problem 26d,e,f
Predict the major product when each reagent reacts with ethylene oxide.
(d) PhNH2 (aniline)
(e) KCN (potassium cyanide)
(f) NaN3 (sodium azide)
Problem 27a,b
Predict the major products of the following reactions, including stereochemistry where appropriate.
a. 2,2-dimethylxirane + H+/H218O (oxygen labeled water)
b. 2,2-dimethylxirane + H18O–/H218O (oxygen labeled water)
Problem 27c,d
Predict the major products of the following reactions, including stereochemistry where appropriate.
(c) (2S,3R)-2-ethyl-2,3-dimethyloxirane + CH3O– / CH3OH
(d) (2S,3R)-2-ethyl-2,3-dimethyloxirane + H+ / CH3OH
Problem 28a,b,c
Give the expected products of the following reactions. Include a protonation step where necessary.
(a) 2,2-dimethyloxirane + isopropylmagnesium bromide
(b) propylene oxide + n-butyllithium
(c) cyclopentyloxirane + ethyllithium
Problem 29d,e,f
Write structural formulas for the following compounds.
(d) divinyl ether
(e) allyl methyl ether
(f) cyclohexene oxide
Problem 29g,h,i
Write structural formulas for the following compounds.
(g) cis-2,3-epoxyhexane
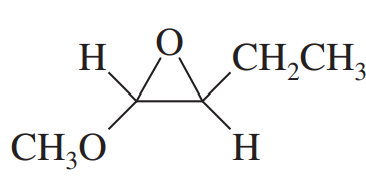
(h) (2R,3S)-2-methoxypentan-3-ol
(i) trans-2,3-dimethyloxirane
Problem 30a-d
Give common names for the following compounds.
(a) (CH3)2CHOCH(CH3)CH2CH3
(b) (CH3)3COCH2CH(CH3)2
(c) PhOCH2CH3
(d) ClCH2OCH2CH2CH3
Problem 30e,f
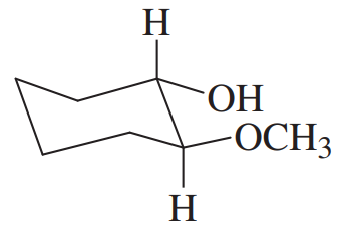
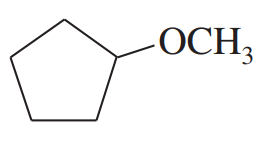
Give common names for the following compounds.
(e)
(f)
Problem 30g
Give common names for the following compounds.
(g)
Problem 31g,h,i
Give IUPAC names for the following compounds.
(g)
(h)
(i)