Back
Back Wade 9th Edition
Wade 9th Edition Ch. 15 - Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy
Ch. 15 - Conjugated Systems, Orbital Symmetry, and Ultraviolet SpectroscopyProblem 1a
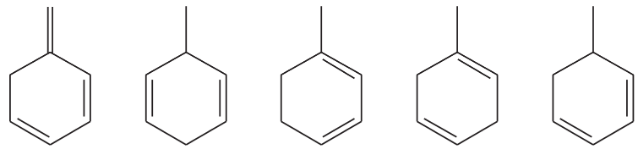
Rank each group of compounds in order of increasing heat of hydrogenation.
(a) hexa-1,2-diene; hexa-1,3,5-triene; hexa-1,3-diene; hexa-1,4-diene; hexa-1,5-diene; hexa-2,4-diene
Problem 1b
Rank each group of compounds in order of increasing heat of hydrogenation.
(b)
Problem 2
In a strongly acidic solution, cyclohexa-1,4-diene tautomerizes to cyclohexa-1,3-diene. Propose a mechanism for this rearrangement, and explain why it is energetically favorable.
Problem 3
The central carbon atom of an allene is a member of two double bonds, and it has an interesting orbital arrangement that holds the two ends of the molecule at right angles to each other.
a. Draw an orbital diagram of allene, showing why the two ends are perpendicular.
b. Draw the two enantiomers of penta-2,3-diene. A model may be helpful.
Problem 4
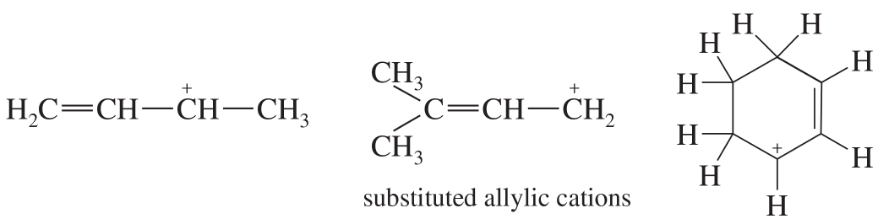
Draw another resonance form for each of the substituted allylic cations shown in the preceding figure, showing how the positive charge is shared by another carbon atom. In each case, state whether your second resonance form is a more important or less important resonance contributor than the first structure. (Which structure places the positive charge on the more-substituted carbon atom?)
Problem 5
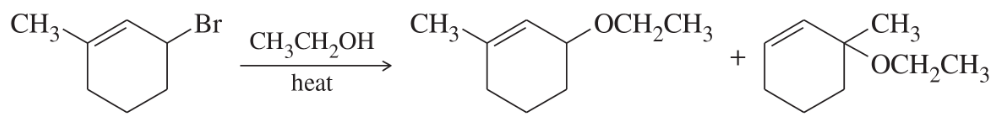
When 3-bromo-1-methylcyclohexene undergoes solvolysis in hot ethanol, two products are formed. Propose a mechanism that accounts for both of these products.
Problem 6
Treatment of an alkyl halide with AgNO3 in alcohol often promotes ionization.
Ag+ + R–Cl → AgCl + R+
When 4-chloro-2-methylhex-2-ene reacts with AgNO3 in ethanol, two isomeric ethers are formed. Suggest structures, and propose a mechanism for their formation
Problem 7b
Propose a mechanism for each reaction, showing explicitly how the observed mixtures of products are formed.
(b) 2-methylbut-3-en-2-ol + HBr → 1-bromo-3-methylbut-2-ene + 3-bromo-3-methylbut-1-ene
Problem 7c
Propose a mechanism for each reaction, showing explicitly how the observed mixtures of products are formed.
c. cyclopenta-1,3-diene + Br2 → 3,4-dibromocyclopent-1-ene + 3,5-dibromocyclopent-1-ene
Problem 7e
Propose a mechanism for each reaction, showing explicitly how the observed mixtures of products are formed.
(e) 3-chlorobut-1-ene + AgNO3, H2O → but-2-en-1-ol + but-3-en-2-ol
Problem 8a,b,c
When Br2 is added to buta-1,3-diene at –15 °C, the product mixture contains 60% of product A and 40% of product B. When the same reaction takes place at 60 °C, the product ratio is 10% A and 90% B.
a. Propose structures for products A and B. (Hint: In many cases, an allylic carbocation is more stable than a bromonium ion.)
b. Propose a mechanism to account for formation of both A and B.
c. Show why A predominates at –15 °C and B predominates at 60 °C.
Problem 8d
When Br2 is added to buta-1,3-diene at –15 °C, the product mixture contains 60% of product A and 40% of product B. When the same reaction takes place at 60 °C, the product ratio is 10% A and 90% B.
d. If you had a solution of pure A, and its temperature were raised to 60 °C, what would you expect to happen? Propose a mechanism to support your prediction.
Problem 10
When N-bromosuccinimide is added to hex-1-ene in CCl4 and a sunlamp is shone on the mixture, three products result.
(a) Give the structures of these three products.
(b) Propose a mechanism that accounts for the formation of these three products
Problem 12
Addition of 1-bromobut-2-ene to magnesium metal in dry ether results in formation of a Grignard reagent. Addition of water to this Grignard reagent gives a mixture of but-1-ene and but-2-ene (cis and trans). When the Grignard reagent is made using 3-bromobut-1-ene, addition of water produces exactly the same mixture of products in the same ratios. Explain this curious result
Problem 13a
Show how you might synthesize the following compounds starting with bromobenzene, and alkyl or alkenyl halides of four carbon atoms or fewer.
a. 3-phenylprop-1-ene
Problem 13b
Show how you might synthesize the following compounds starting with bromobenzene, and alkyl or alkenyl halides of four carbon atoms or fewer.
b. 5-methylhex-2-ene
Problem 13c
Show how you might synthesize the following compounds starting with bromobenzene, and alkyl or alkenyl halides of four carbon atoms or fewer.
c. dec-5-ene
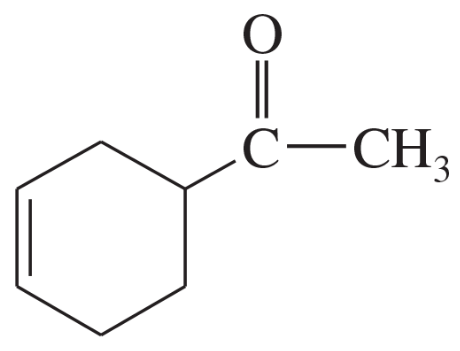
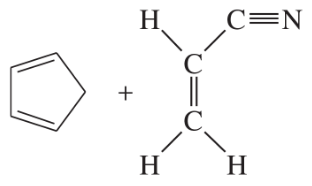
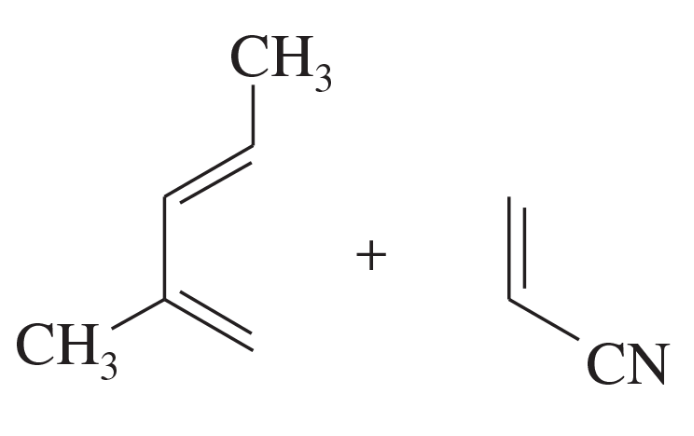
Problem 14a
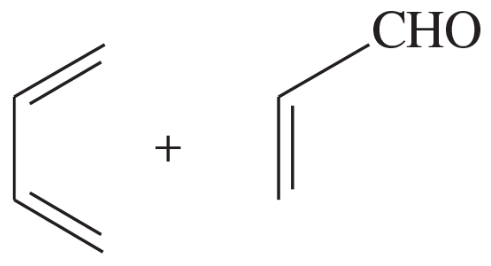
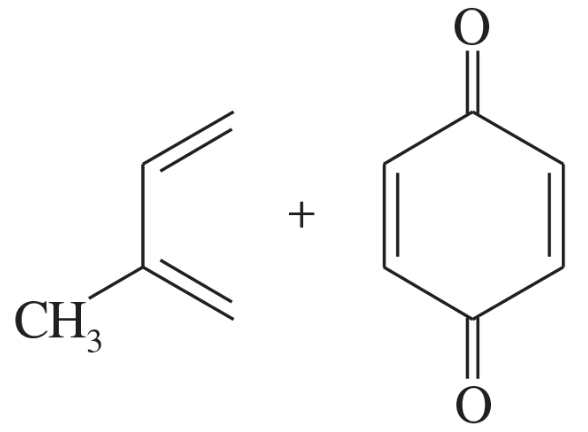
Predict the products of the following proposed Diels–Alder reactions.
(a)
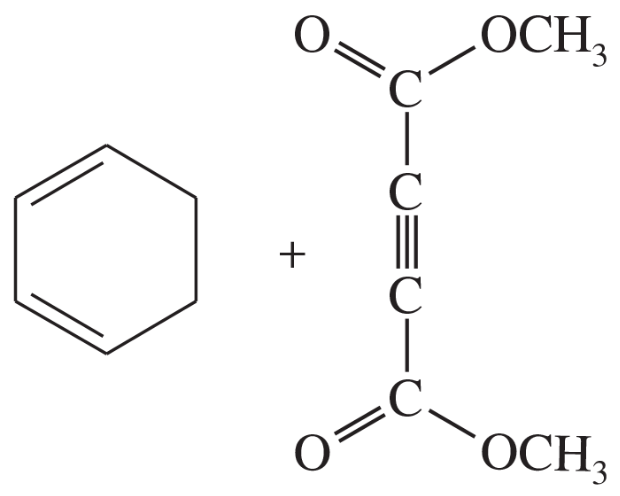
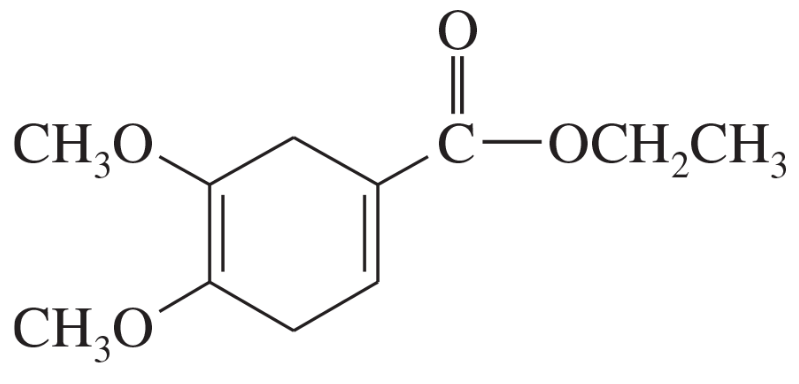
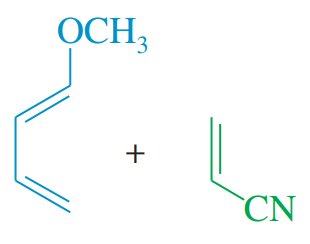
Problem 14b
Predict the products of the following proposed Diels–Alder reactions.
(b)
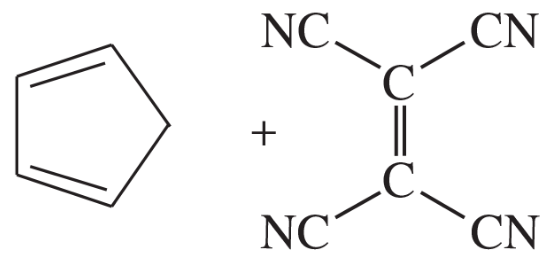
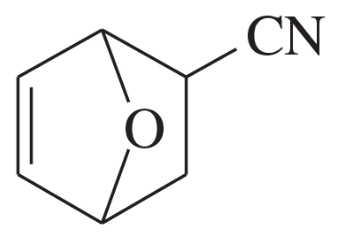
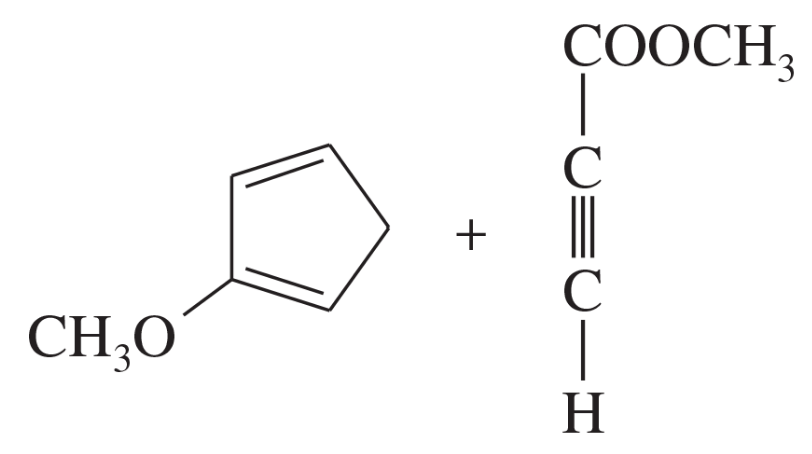
Problem 14c
Predict the products of the following proposed Diels–Alder reactions.
(c)
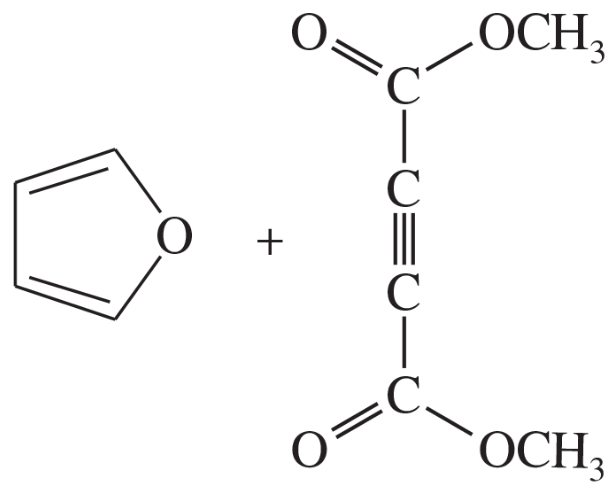
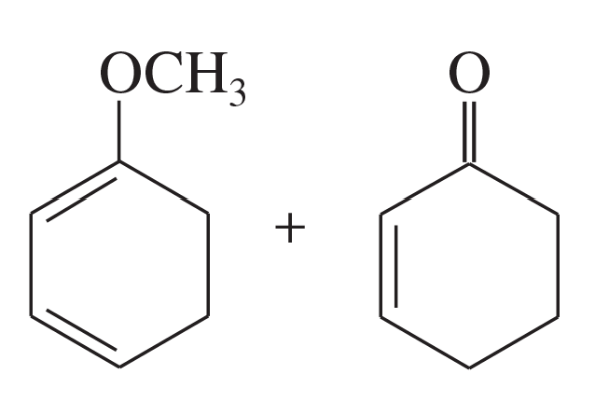
Problem 14d
Predict the products of the following proposed Diels–Alder reactions.
(d)
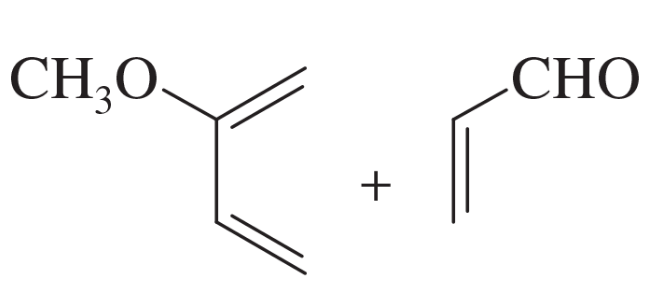
Problem 14e
Predict the products of the following proposed Diels–Alder reactions.
(e)
Problem 14f
Predict the products of the following proposed Diels–Alder reactions.
(f)
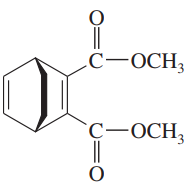
Problem 15a,b,c
What dienes and dienophiles would react to give the following Diels–Alder products?
(a)
(b)
(c)
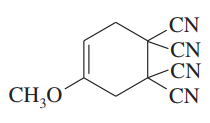
Problem 15d,e,f
What dienes and dienophiles would react to give the following Diels–Alder products?
(d)
(e)
(f)
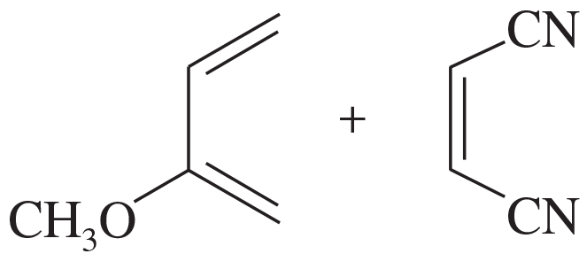
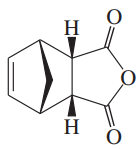
Problem 16a
Predict the major product for each proposed Diels–Alder reaction. Include stereochemistry where appropriate.
(a)
Problem 17a,b
We predicted that the products would have a 1,2- or 1,4-relationship of the proper substituents. Draw the charge-separated resonance forms of the reactants to support these predictions.
(a)
(b)
Problem 18a,b
Predict the products of the following Diels–Alder reactions.
(a)
(b)
Problem 18c,d
Predict the products of the following Diels–Alder reactions.
(c)
(d)
Problem 19
Show that the [4 + 2] Diels–Alder reaction is photochemically forbidden.