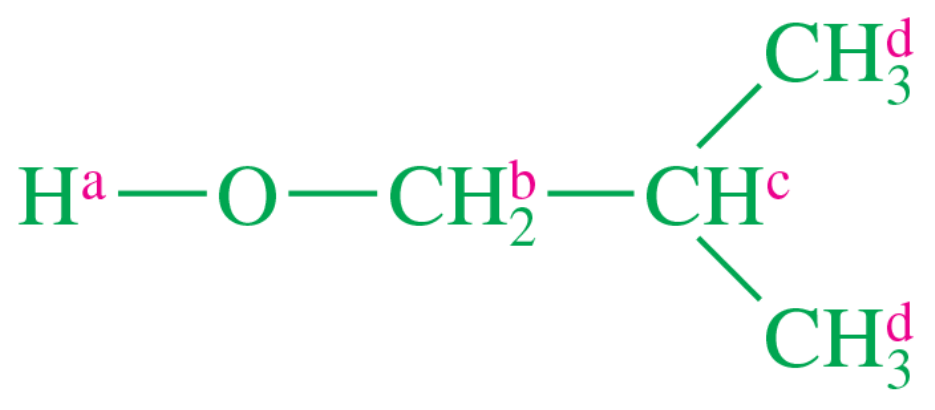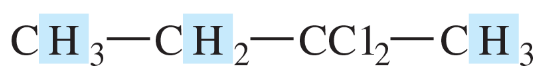Back
BackProblem 1
In a 300-MHz spectrometer, the protons in iodomethane absorb at a position 650 Hz downfield from TMS.
(a) What is the chemical shift of these protons?
(b) What is the chemical shift of the iodomethane protons in a 60-MHz spectrometer?
(c) How many hertz downfield from TMS would they absorb at 60 MHz?
Problem 2a,b
Predict the chemical shifts of the protons in the following compounds.
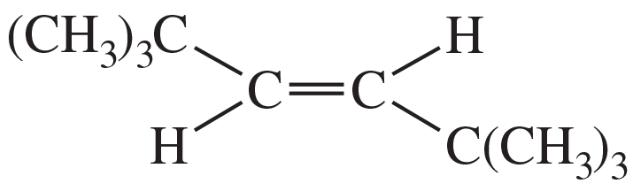
(a)
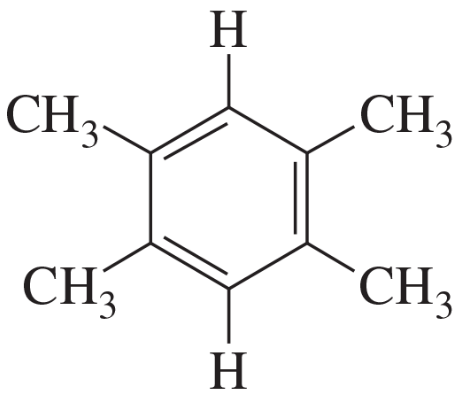
(b)
Problem 3a,b,c
Determine the number of different kinds of protons in each compound.
(a) 1-chloropropane
(b) 2-chloropropane
(c) 2,2-dimethylbutane
Problem 4
The NMR spectrum of toluene (methylbenzene) was shown in Figure 13-11.
<IMAGE>
(a) How many different kinds of protons are there in toluene?
(b) Explain why the aromatic region around d7.2 is broad, with more than one sharp absorption.
Problem 8a
Draw the NMR spectra you expect for the following compounds.
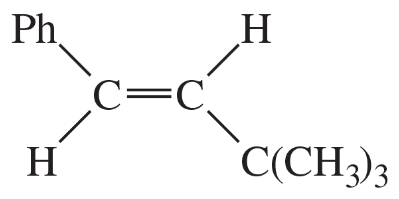
(a)
Problem 8b
Draw the NMR spectra you expect for the following compounds.
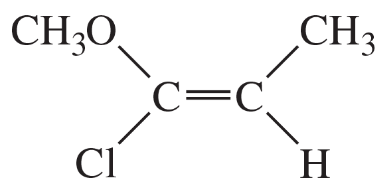
(b)
Problem 10
An unknown compound (C3H2NCl) shows moderately strong IR absorptions around 1650 cm–1 and 2200 cm–1. Its NMR spectrum consists of two doublets (J = 14 Hz) at δ5.9 and δ7.1. Propose a structure consistent with these data.
Problem 11a
Propose a structure that corresponds to each spectrum.
(a) <IMAGE>
Problem 12
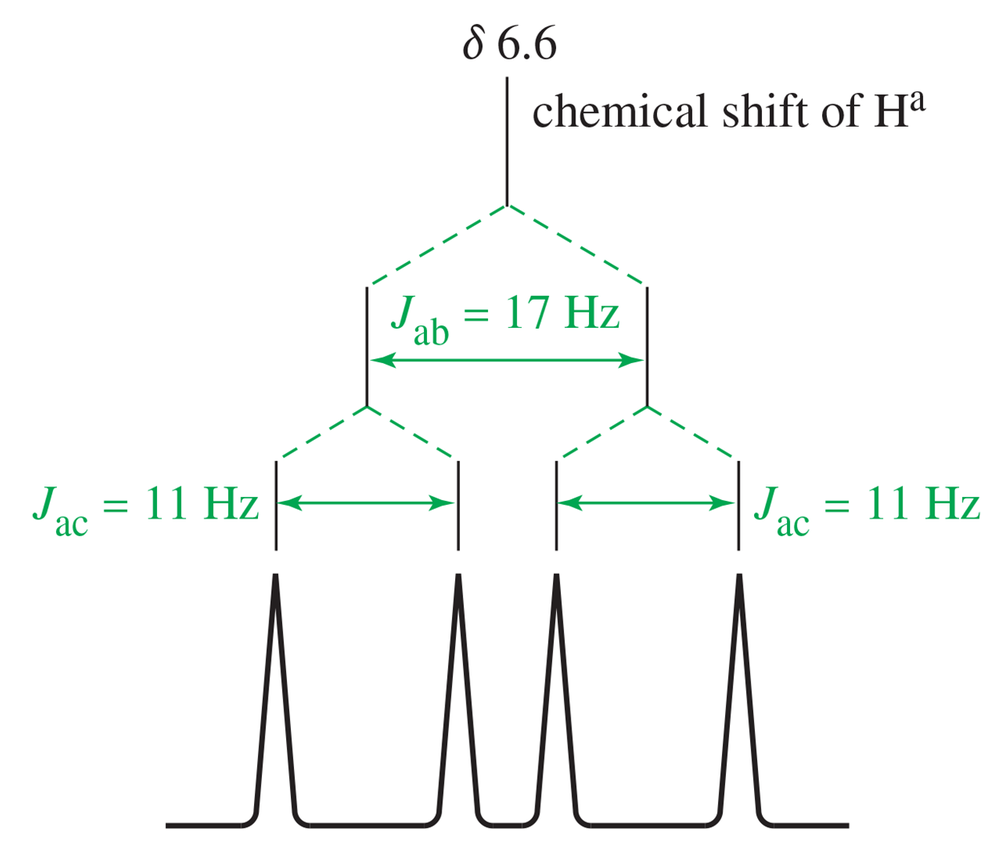
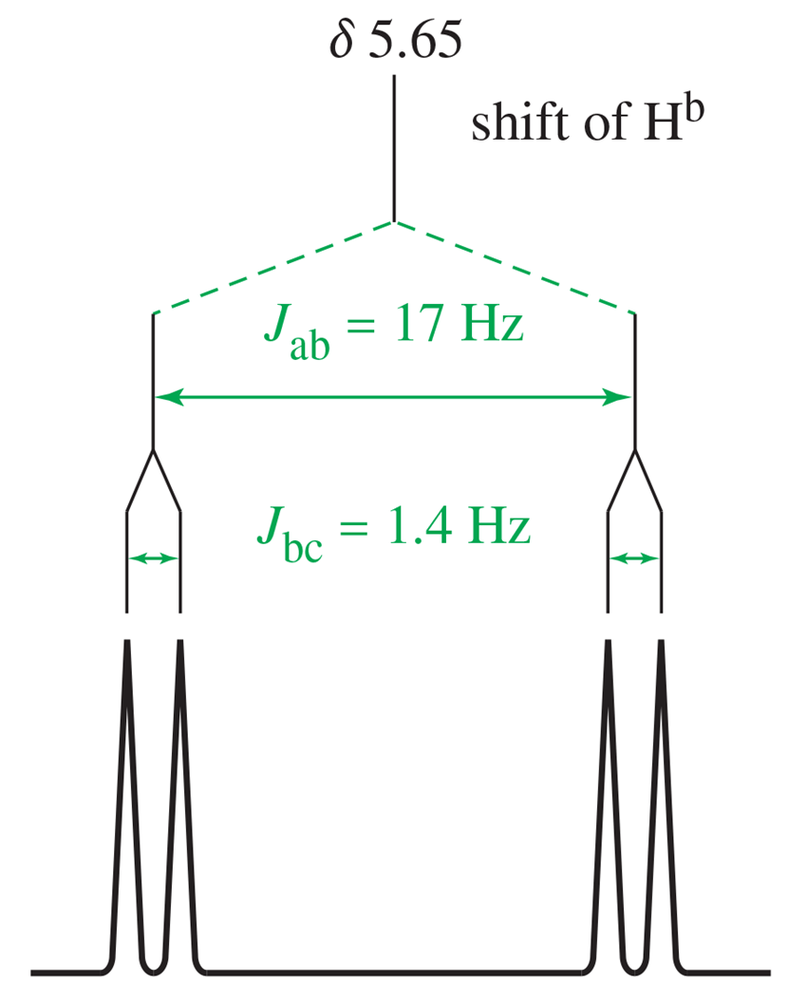
Draw a splitting tree, similar to Figures 13-32 and 13-33, for proton Hc in styrene. What is the chemical shift of proton Hc?
Problem 13a
The spectrum of trans-hex-2-enoic acid follows.
(a) Assign peaks to show which protons give rise to which peaks in the spectrum.
(b) Draw a tree to show the complex splitting of the vinyl proton centered around 7 ppm. Estimate the values of the coupling constants.
Problem 17a
If the imaginary replacement of either of two protons forms enantiomers, then those protons are said to be enantiotopic. The NMR is not a chiral probe, and it cannot distinguish between enantiotopic protons. They are seen to be “equivalent by NMR.”
(a) Use the imaginary replacement technique to show that the two allylic protons (those on C3) of allyl bromide are enantiotopic.
Problem 18a,b
Predict the theoretical number of different NMR signals produced by each compound, and give approximate chemical shifts. Point out any diastereotopic relationships.
a. 2-bromobutane
b. cyclopentanol
Problem 18c,d
Predict the theoretical number of different NMR signals produced by each compound, and give approximate chemical shifts. Point out any diastereotopic relationships.
c. Ph—CHBr—CH2Br
d. vinyl chloride
Problem 19a,b
Propose mechanisms to show the interchange of protons between ethanol molecules under
(a) acid catalysis.
(b) base catalysis.
Problem 20a
Draw the NMR spectrum expected from ethanol that has been shaken with a drop of D2O.
Problem 21a
Propose chemical structures consistent with the following NMR spectra and molecular formulas. In spectrum (a), explain why the peaks around δ1.65 and δ3.75 are not clean multiplets, but show complex splitting.
(a) <IMAGE>
Problem 22
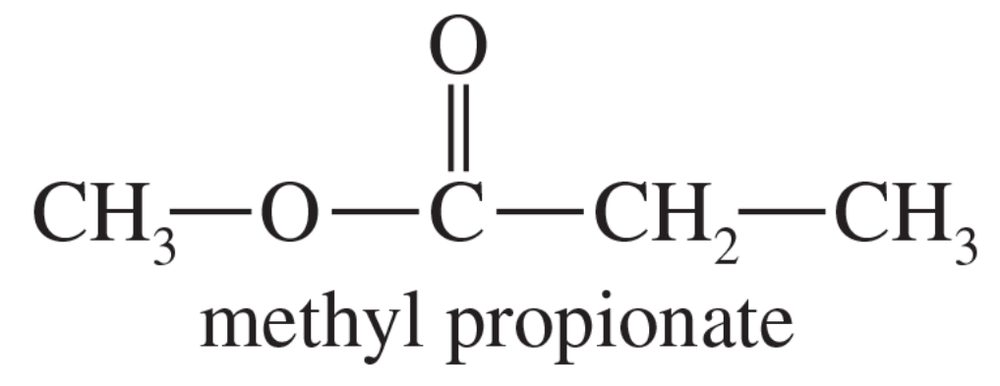
Draw the expected NMR spectrum of methyl propionate, and point out how it differs from the spectrum of ethyl acetate.
Problem 23
Give the spectral assignments for the protons in isobutyl alcohol (Solved Problem 13-4). For example, Ha is a singlet, area = 1, at δ2.4.
Problem 24b
Five proton NMR spectra are given here, together with molecular formulas. In each case, propose a structure that is consistent with the spectrum.
(b) <IMAGE>
Problem 26a
(a) Show which carbon atoms correspond with which peaks in the 13C NMR spectrum of butan-2-one (Figure 13-45).
<IMAGE>
Problem 26b
(b) Draw the proton NMR spectrum you would expect for butan-2-one. How well do the proton chemical shifts predict the carbon chemical shifts using the "15 to 20 times as large" rule of thumb?
Problem 28
The standard 13C NMR spectrum of phenyl propanoate is shown here. Predict the appearance of the DEPT-90 and DEPT-135 spectra.
<IMAGE>
Problem 29
A bottle of allyl bromide was found to contain a large amount of an impurity. A careful distillation separated the impurity, which has the molecular formula C3H6O. The following 13C NMR spectrum of the impurity was obtained:
<IMAGE>
(a) Propose a structure for this impurity.
(b) Assign the peaks in the 13C NMR spectrum to the carbon atoms in the structure.
(c) Suggest how this impurity arose in the allyl bromide sample.
Problem 30
An inexperienced graduate student was making some 4-hydroxybutanoic acid. He obtained an excellent yield of a different compound, whose 13C NMR spectrum is shown here.
<IMAGE>
(a) Propose a structure for this product.
(b) Assign the peaks in the 13C NMR spectrum to the carbon atoms in the structure.
Problem 31
A laboratory student was converting cyclohexanol to cyclohexyl bromide by using one equivalent of sodium bromide in a large excess of concentrated sulfuric acid. The major product she recovered was not cyclohexyl bromide, but a compound of formula C6H10 that gave the following 13C NMR spectrum:
<IMAGE>
(a) Propose a structure for this product.
(b) Assign the peaks in the 13C NMR spectrum to the carbon atoms in the structure.
(c) Suggest modifications in the reaction to obtain a better yield of cyclohexyl bromide.
Problem 33
An unknown compound has the molecular formula C9H11Br. Its proton NMR spectrum shows the following absorptions:
singlet, δ7.1, integral 44 mm
singlet, δ2.3, integral 130 mm
singlet, δ2.2, integral 67 mm
Propose a structure for this compound.
Problem 34a,b
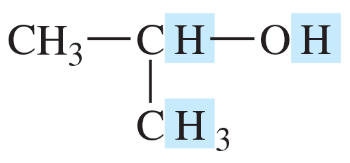
Predict the multiplicity (the number of peaks as a result of splitting) and the chemical shift for each shaded proton in the following compounds.
(a)
(b)
Problem 35c,d
Predict the approximate chemical shifts of the protons in the following compounds.
(c) CH3–O–CH2CH2CH2Cl
(d) CH3CH2–C≡C–H
Problem 36
The following proton NMR spectrum is of a compound of molecular formula C3H8O.
<IMAGE>
(a) Propose a structure for this compound.
(b) Assign peaks to show which protons give rise to which signals in the spectrum.
Problem 37
Using a 60-MHz spectrometer, a chemist observes the following absorption: doublet, J = 7 Hz, at δ4.00
(a) What would the chemical shift (δ) be in the 300-MHz spectrum?
(b) What would the splitting value J be in the 300-MHz spectrum?
(c) How many hertz from the TMS peak is this absorption in the 60-MHz spectrum? In the 300-MHz spectrum?