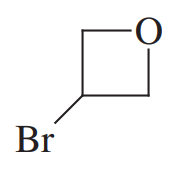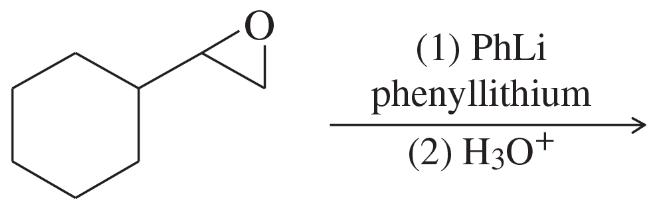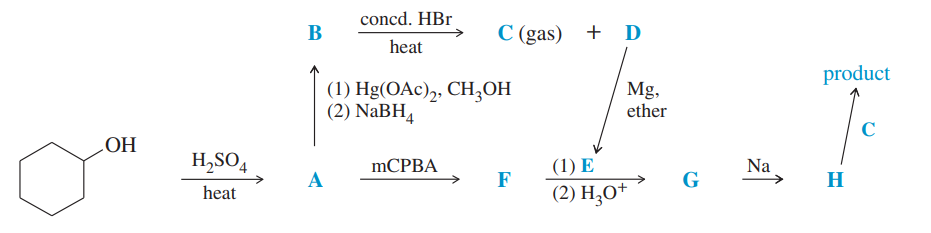Back
BackProblem 24a
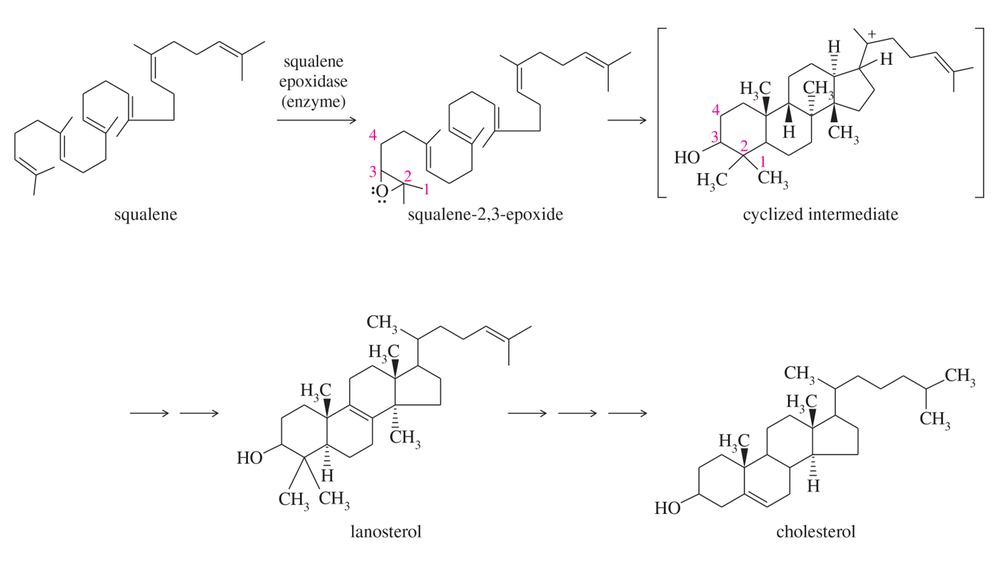
Show the rest of the mechanism for formation of the cyclized intermediate in Figure 14-6.
Problem 26a,b,c
Predict the major product when each reagent reacts with ethylene oxide.
(a) NaOCH2CH3 (sodium ethoxide)
(b) NaNH2 (sodium amide)
(c) NaSPh (sodium thiophenoxide)
Problem 26d,e,f
Predict the major product when each reagent reacts with ethylene oxide.
(d) PhNH2 (aniline)
(e) KCN (potassium cyanide)
(f) NaN3 (sodium azide)
Problem 27a,b
Predict the major products of the following reactions, including stereochemistry where appropriate.
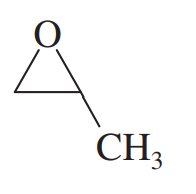
a. 2,2-dimethylxirane + H+/H218O (oxygen labeled water)
b. 2,2-dimethylxirane + H18O–/H218O (oxygen labeled water)
Problem 27c,d
Predict the major products of the following reactions, including stereochemistry where appropriate.
(c) (2S,3R)-2-ethyl-2,3-dimethyloxirane + CH3O– / CH3OH
(d) (2S,3R)-2-ethyl-2,3-dimethyloxirane + H+ / CH3OH
Problem 28a,b,c
Give the expected products of the following reactions. Include a protonation step where necessary.
(a) 2,2-dimethyloxirane + isopropylmagnesium bromide
(b) propylene oxide + n-butyllithium
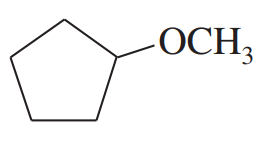
(c) cyclopentyloxirane + ethyllithium
Problem 29d,e,f
Write structural formulas for the following compounds.
(d) divinyl ether
(e) allyl methyl ether
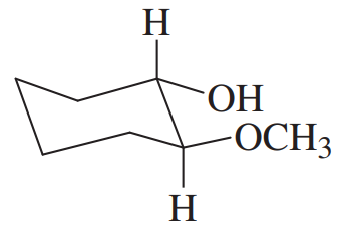
(f) cyclohexene oxide
Problem 29g,h,i
Write structural formulas for the following compounds.
(g) cis-2,3-epoxyhexane
(h) (2R,3S)-2-methoxypentan-3-ol
(i) trans-2,3-dimethyloxirane
Problem 30a-d
Give common names for the following compounds.
(a) (CH3)2CHOCH(CH3)CH2CH3
(b) (CH3)3COCH2CH(CH3)2
(c) PhOCH2CH3
(d) ClCH2OCH2CH2CH3
Problem 30e,f
Give common names for the following compounds.
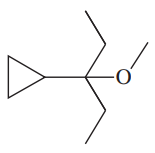
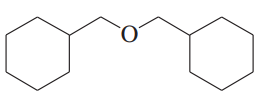
(e)
(f)
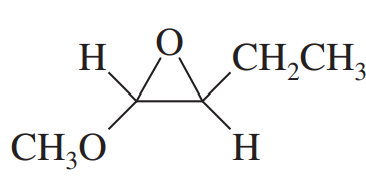
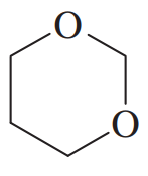
Problem 30g
Give common names for the following compounds.
(g)
Problem 31g,h,i
Give IUPAC names for the following compounds.
(g)
(h)
(i)
Problem 33a,b,c
Predict the products of the following reactions.
(a) sec-butyl isopropyl ether + concd. HBr, heat
(b) 2-ethoxy-2-methylpentane + concd. HBr, heat
(c) di-n-butyl ether + hot concd. NaOH
Problem 33g,h
Predict the products of the following reactions.
(g) trans-2,3-epoxyoctane + H+, H2O
(h) propylene oxide + methylamine (CH3NH2)
Problem 33j,k,l
Predict the products of the following reactions.
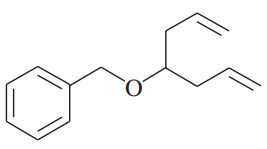
(j)
(k)
(l)
Problem 33m,n
Predict the products of the following reactions.
(m)
(n)
Problem 34a,b,c
Show how you would make the following ethers, using only simple alcohols and any needed reagents as your starting materials.
(a) 1-methoxybutane
(b) 2-ethoxy-2-methylpropane
(c) benzyl cyclopentyl ether
Problem 35
(A true story.) An inexperienced graduate student moved into a laboratory and began work. He needed some diethyl ether for a reaction, so he opened an old, rusty 1-gallon can marked 'ethyl ether' and found there was half a gallon left. To purify the ether, the student set up a distillation apparatus, started a careful distillation, and went to the stockroom for the other reagents he needed. While he was at the stockroom, the student heard a muffled "boom." He quickly returned to his lab to find a worker from another laboratory putting out a fire. Most of the distillation apparatus was embedded in the ceiling.
(a) Explain what probably happened.
(b) Explain how this near-disaster might have been prevented.
Problem 37
(a) Show how you would synthesize the pure (R) enantiomer of 2-butyl methyl sulfide, starting with pure (R)-butan-2-ol and any reagents you need
(b) Show how you would synthesize the pure (S) enantiomer of the product, still starting with (R)-butan-2-ol and any reagents you need.
Problem 39
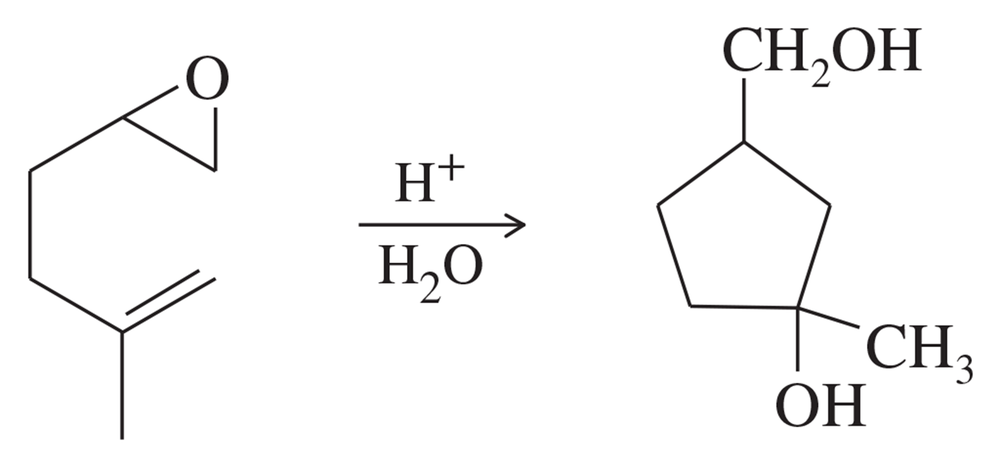
The following reaction resembles the acid-catalyzed cyclization of squalene oxide. Propose a mechanism for this reaction.
Problem 40a,b,c
Show how you would convert hex-1-ene to each of the following compounds. You may use any additional reagents and solvents you need.
(a) 2-methoxyhexane
(b) 1-methoxyhexane
(c) 1-methoxyhexan-2-ol
Problem 41a
Both LiAlH4 and Grignard reagents react with carbonyl compounds to give alkoxide ion intermediates (that become protonated in an aqueous workup). Those alkoxides can react with 1° or methyl alkyl halides or tosylates to give ethers. Show how the following ethers can be formed in this two-step process. As starting materials you may use any reactants containing 7 carbons or fewer.
(a)
Problem 41b
Both LiAlH4 and Grignard reagents react with carbonyl compounds to give alkoxide ion intermediates (that become protonated in an aqueous workup). Those alkoxides can react with 1° or methyl alkyl halides or tosylates to give ethers. Show how the following ethers can be formed in this two-step process. As starting materials you may use any reactants containing 7 carbons or fewer.
(b)
Problem 41c
Both LiAlH4 and Grignard reagents react with carbonyl compounds to give alkoxide ion intermediates (that become protonated in an aqueous workup). Those alkoxides can react with 1° or methyl alkyl halides or tosylates to give ethers. Show how the following ethers can be formed in this two-step process. As starting materials you may use any reactants containing 7 carbons or fewer.
(c)
Problem 42
Give the structures of intermediates A through H in the following synthesis of trans-1-cyclohexyl-2-methoxycyclohexane.
Problem 43a
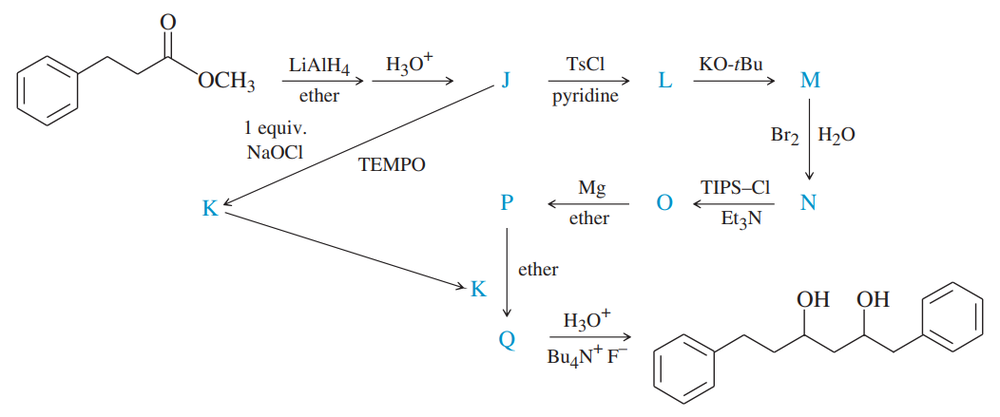
Give the structures of the intermediates represented by letters J, L, M, and N in this synthesis.
Problem 43b
Give the structures of the intermediates represented by letters K and Q in this synthesis.
Problem 43c
Give the structures of the intermediates represented by letters O, P, and Q in this synthesis.
Problem 45a
Show how you would synthesize the following ethers in good yield from the indicated starting materials and any additional reagents needed.
(a) cyclopentyl n-propyl ether from cyclopentanol and propan-1-ol
Problem 45b
Show how you would synthesize the following ethers in good yield from the indicated starting materials and any additional reagents needed.
(b) n-butyl phenyl ether from phenol and butan-1-ol