Back
BackProblem 1
Rank the given solvents in decreasing order of their ability to dissolve each compound.
Solutes:
(a) NaOAc
(b)
(c)
Solvents:
ethyl ether
water
ethanol
dichloromethane
Problem 2
Aluminum trichloride (AlCl3) dissolves in ether with the evolution of a large amount of heat. (In fact, this reaction can become rather violent if it gets too warm.) Show the structure of the resulting aluminum chloride etherate complex.
Problem 3
In the presence of 18-crown-6, potassium permanganate dissolves in benzene to give "purple benzene," a useful reagent for oxidizing alkenes in an aprotic environment. Use a drawing of the complex to show why KMnO4 dissolves in benzene and why the reactivity of the permanganate ion is enhanced.
Problem 4a,b,c
Give a common name (when possible) and a systematic name for each compound.
(a) CH3OCH=CH2
(b) CH3CH2OCH(CH3)2
(c) ClCH2CH2OCH3
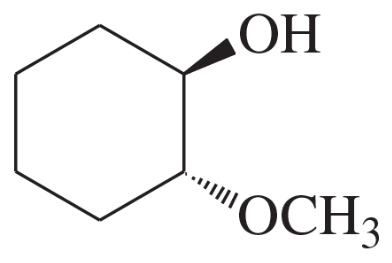
Problem 4d,e,f
Give a common name (when possible) and a systematic name for each compound.
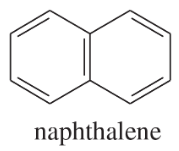
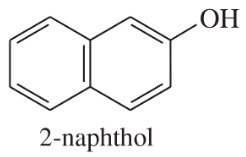
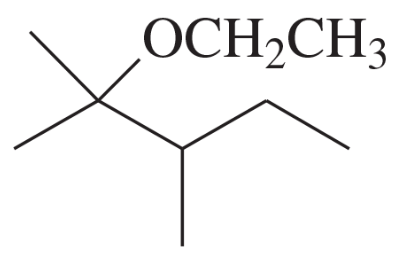
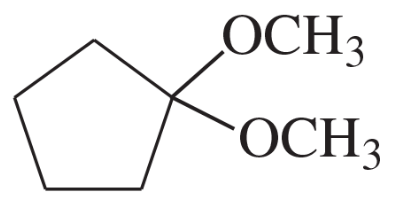
(d)
(e)
(f)
Problem 5
1,4-Dioxane is made commercially by the acid-catalyzed condensation of an alcohol.
(a) Show what alcohol will undergo condensation, with loss of water, to give 1,4-dioxane.
(b) Propose a mechanism for this reaction.
Problem 7
Propose a fragmentation to account for each numbered peak in the mass spectrum of n-butyl isopropyl ether.
<IMAGE>
Problem 8
Propose a Williamson synthesis of 3-butoxy-1,1-dimethylcyclohexane from 3,3-dimethyl-cyclohexanol and butan-1-ol.
Problem 9d,e
Show how you would use the Williamson ether synthesis to prepare the following ethers. You may use any alcohols or phenols as your organic starting materials.
(d) ethyl n-propyl ether (two ways)
(e) benzyl tert-butyl ether (benzyl = Ph–CH2–)
Problem 10a,b,c
Show how the following ethers might be synthesized using (1) alkoxymercuration– demercuration and (2) the Williamson synthesis. (When one of these methods cannot be used for the given ether, point out why it will not work.)
a. 2-methoxybutane
b. ethyl cyclohexyl ether
c. 1-methoxy-2-methylcyclopentane
Problem 10d
Show how the following ethers might be synthesized using (1) alkoxymercuration– demercuration and (2) the Williamson synthesis. (When one of these methods cannot be used for the given ether, point out why it will not work.)
(d) 1-methoxy-1-methylcyclopentane
Problem 10e
Show how the following ethers might be synthesized using (1) alkoxymercuration– demercuration and (2) the Williamson synthesis. (When one of these methods cannot be used for the given ether, point out why it will not work.)
(e) 1-isopropoxy-1-methylcyclopentane