Back
Back Mullins 1st Edition
Mullins 1st Edition Ch. 3 - Alkanes and Cycloalkanes: Properties and Conformational Analysis
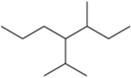
Ch. 3 - Alkanes and Cycloalkanes: Properties and Conformational AnalysisProblem 15k
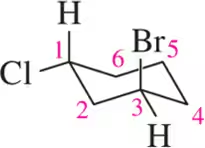
Name the following alkanes using the IUPAC system of nomenclature. [Each molecule exemplifies one of the nomenclature rules in Tables 3.7 and 3.8.]
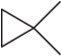
(k) rule 6
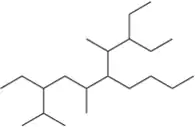
Problem 15n
Name the following alkanes using the IUPAC system of nomenclature. [Each molecule exemplifies one of the nomenclature rules in Tables 3.7 and 3.8.]
(n) rule 7
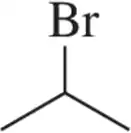
Problem 16b
When we begin studying functional groups, the designation of substitution will be especially important. Label the following bromoalkanes as 1° , 2°, 3°, or 4° based on the carbon to which the bromine is attached.
(b)
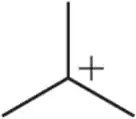
Problem 17b
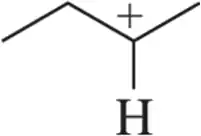
When we begin studying reactive intermediates, the designation of substitution will be especially important. Label the following carbocations as 1° , 2°, 3°, or 4° or based on the carbon bearing the positive charge.
(b)
Problem 17c
When we begin studying reactive intermediates, the designation of substitution will be especially important. Label the following carbocations as 1° , 2°, 3°, or 4° or based on the carbon bearing the positive charge.
(c)
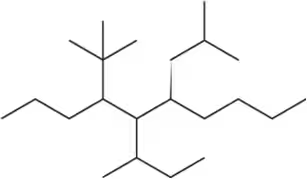
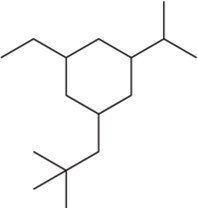
Problem 19c
Name the following alkanes using the common names for branched substituents.
(c)
Problem 20
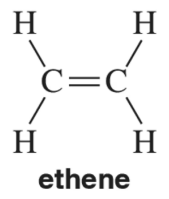
In Chapter 8, we study the chemistry of alkenes, like ethene. In contrast to ethane, there is no free rotation around the C = C double bond of ethene. Explain this in the context of the molecular orbital picture of ethene.
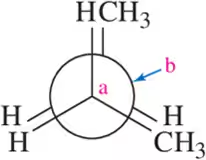
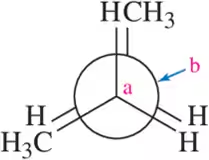
Problem 21d
For each molecule, draw the Newman projection you would observe if you looked down the Ca - Cb bond in the direction indicated.
(d) <IMAGE>
Problem 22b
Using the Newman projections shown, draw each molecule in its line-angle drawing with all hydrogens and substituents shown. [Carbon b is behind carbon a in these structures.] Wedges and dashes should be used to indicate whether a substituent is coming out of, or going into, the plane of the page.
(b)
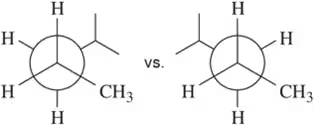
Problem 22d
Using the Newman projections shown, draw each molecule in its line-angle drawing with all hydrogens and substituents shown. [Carbon b is behind carbon a in these structures.] Wedges and dashes should be used to indicate whether a substituent is coming out of, or going into, the plane of the page.
(d)
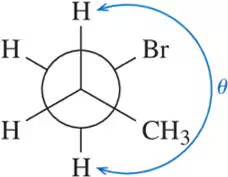
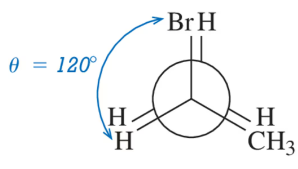
Problem 23(c)
Calculate the dihedral angle (θ) for the conformations shown.
(c)
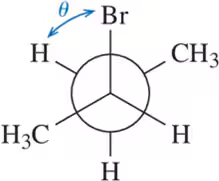
Problem 23a
Calculate the dihedral angle (θ) for the conformations shown.
(a)
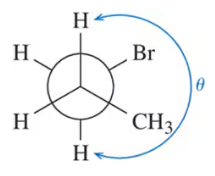
Problem 23c
Calculate the dihedral angle (θ) for the conformations shown.
(c)
Problem 24
Why is it incorrect to say that the dihedral angle shown is 120° or even 109°?
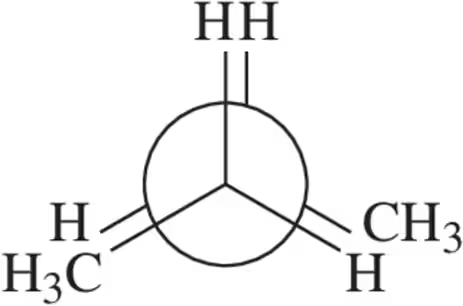
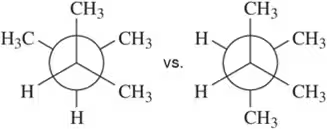
Problem 26a
Calculate the total strain in the eclipsed conformations shown.
(a)
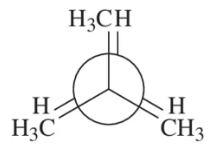
Problem 26b
Calculate the total strain in the eclipsed conformations shown.
(b)
Problem 27
Would you expect a CH3/CH3 eclipsing interaction to be larger or smaller than 1.3 kcal/mol?
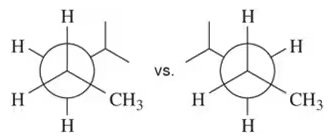
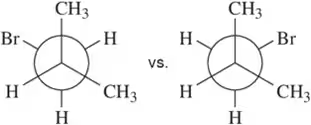
Problem 29a
Choose the conformation in each pair that is most stable. If both are equally stable, then write 'no difference.'
(a)
Problem 30
For each of the pairs in Assessment 3.29, which conformation would you expect to be more prominent at equilibrium?
(a)
(b)
(c)
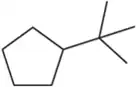
Problem 32a
Name the following cycloalkanes using the IUPAC system of nomenclature. [Hint: Each molecule exemplifies one of the cycloalkane nomenclature rules in Table 3.10.]
(a) rule 1
Problem 32c
Name the following cycloalkanes using the IUPAC system of nomenclature. [Hint: Each molecule exemplifies one of the cycloalkane nomenclature rules in Table 3.10.]
(c) rule 2
Problem 32e
Name the following cycloalkanes using the IUPAC system of nomenclature. [Hint: Each molecule exemplifies one of the cycloalkane nomenclature rules in Table 3.10.]
(e) rule 3
Problem 32g
Name the following cycloalkanes using the IUPAC system of nomenclature. [Hint: Each molecule exemplifies one of the cycloalkane nomenclature rules in Table 3.10.]
(g) rule 4
Problem 33
Name the following cycloalkanes using the IUPAC system of nomenclature.
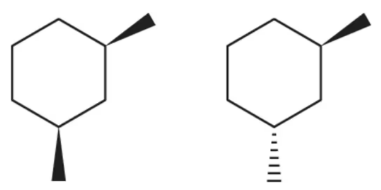
Problem 34b
Name the following cycloalkanes using the IUPAC system of nomenclature, being sure to indicate whether they are cis or trans.
(b)
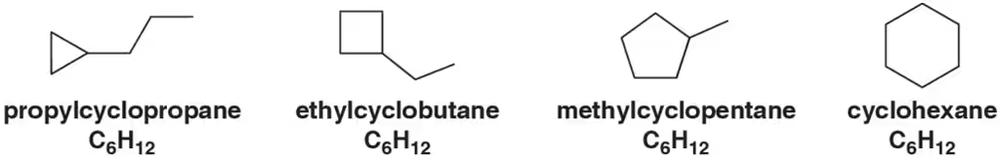
Problem 36
Rank the following compounds in order of their total heat of combustion. These compounds are constitutional isomers, each with a molecular formula of C6H12.
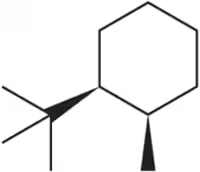
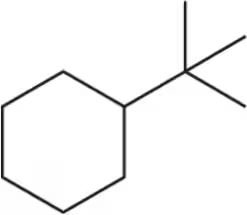
Problem 39a
Draw two chair conformations of the molecules below. Indicate which is most stable.
(a)
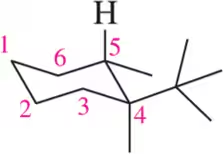
Problem 40b
Given the following chair conformations, draw each in its planar form as if you were viewing it from above.
(b)
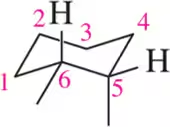
Problem 40c
Given the following chair conformations, draw each in its planar form as if you were viewing it from above.
(c)
Problem 40d
Given the following chair conformations, draw each in its planar form as if you were viewing it from above.
(d)