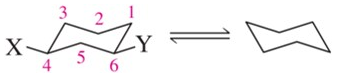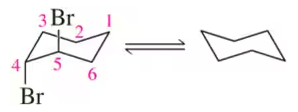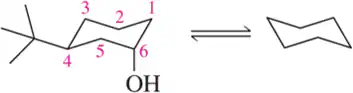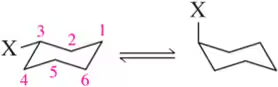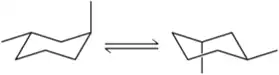Back
Back Mullins 1st Edition
Mullins 1st Edition Ch. 3 - Alkanes and Cycloalkanes: Properties and Conformational Analysis
Ch. 3 - Alkanes and Cycloalkanes: Properties and Conformational AnalysisProblem 47d
Represent each of the following condensed structural formulas using a line-angle drawing.
(d) CH3CH2CH2CCH2OH
Problem 47e
Represent each of the following condensed structural formulas using a line-angle drawing.
(e) (CH3)2CHCH2CH2CH2CH2CHO
Problem 48b
For each molecular formula, represent all constitutional isomers using line-angle drawings.
(b) C3H8
Problem 48c
For each molecular formula, represent all constitutional isomers using line-angle drawings.
(c) C4H10
Problem 48e
For each molecular formula, represent all constitutional isomers using line-angle drawings.
(e) C6H14
Problem 49a
Draw in all missing lone pairs for the following molecules.
(a)
Problem 49b
Draw in all missing lone pairs for the following molecules.
(b)
Problem 49c
Draw in all missing lone pairs for the following molecules.
(c)
Problem 49d
Draw in all missing lone pairs for the following molecules.
(d)
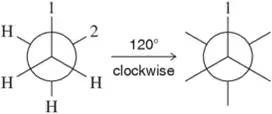
Problem 50a
Given the first Newman projection and the direction and degree of rotation, fill in the resulting Newman projection. [One substituent has been labeled for you.]
(a)
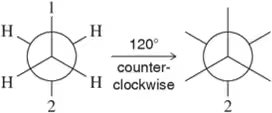
Problem 50d
Given the first Newman projection and the direction and degree of rotation, fill in the resulting Newman projection. [One substituent has been labeled for you.]
(d)
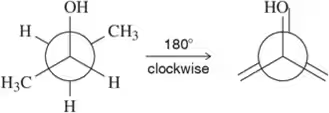
Problem 50h
Given the first Newman projection and the direction and degree of rotation, fill in the resulting Newman projection. [One substituent has been labeled for you.]
(h)
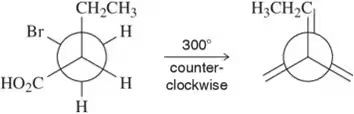
Problem 50l
Given the first Newman projection and the direction and degree of rotation, fill in the resulting Newman projection. [One substituent has been labeled for you.]
(l)
Problem 52d
For each of the following structures, which staggered Newman projection skeleton from Assessment 3.51 should you draw first to show what is seen when looking down the indicated bond?
(d) <IMAGE>
Problem 53b
Given the following structures, show the Newman projection that would result from looking down the indicated bond in the direction shown. [Orient yourself as if you were the eyeball looking down the bond. Some of the examples have been partially completed for you to fill in the rest.]
(b) <IMAGE>
Problem 53i
Given the following structures, show the Newman projection that would result from looking down the indicated bond in the direction shown. [Orient yourself as if you were the eyeball looking down the bond. Some of the examples have been partially completed for you to fill in the rest.]
(i) <IMAGE>
Problem 53j
Given the following structures, show the Newman projection that would result from looking down the indicated bond in the direction shown. [Orient yourself as if you were the eyeball looking down the bond. Some of the examples have been partially completed for you to fill in the rest.]
(j) <IMAGE>
Problem 53k
Given the following structures, show the Newman projection that would result from looking down the indicated bond in the direction shown. [Orient yourself as if you were the eyeball looking down the bond. Some of the examples have been partially completed for you to fill in the rest.]
(k) <IMAGE>
Problem 53l
Given the following structures, show the Newman projection that would result from looking down the indicated bond in the direction shown. [Orient yourself as if you were the eyeball looking down the bond. Some of the examples have been partially completed for you to fill in the rest.]
(l) <IMAGE>
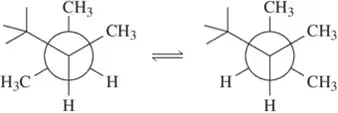
Problem 54e
For each pair of conformations shown, choose which is most stable. [If both conformations have the same number of gauche interactions, choose the one where the interactions are between smaller groups.]
(e)
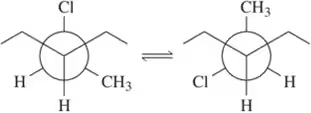
Problem 54f
For each pair of conformations shown, choose which is most stable. [If both conformations have the same number of gauche interactions, choose the one where the interactions are between smaller groups.]
(f)
Problem 55b
Looking down the indicated bond, show the three most stable conformations and choose the one that is most stable. Be sure that the first Newman projection you show is the one you see initially (before rotation). [Why should none of your three Newman projections show eclipsed conformations?]
(b) <IMAGE>
Problem 55e
Looking down the indicated bond, show the three most stable conformations and choose the one that is most stable. Be sure that the first Newman projection you show is the one you see initially (before rotation). [Why should none of your three Newman projections show eclipsed conformations?]
(e) <IMAGE>
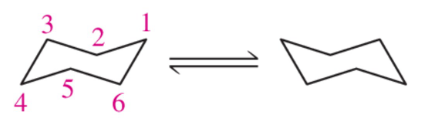
Problem 56
Using the numbers shown in the chair conformation on the left, label the carbons of the flipped chair on the right. [Assume that the angle through which you view the chair conformation doesn't change.]
Problem 57c
For each chair on the left, place the substituents on the flipped chair. [Recall that the axial/equatorial designation changes from one chair to the next, but the carbon to which the substituent is attached does not.]
(c)
Problem 57e
For each chair on the left, place the substituents on the flipped chair. [Recall that the axial/equatorial designation changes from one chair to the next, but the carbon to which the substituent is attached does not.]
(e)
Problem 57f
For each chair on the left, place the substituents on the flipped chair. [Recall that the axial/equatorial designation changes from one chair to the next, but the carbon to which the substituent is attached does not.]
(f)
Problem 58a
What is the mistake that was made in drawing each of the flipped chairs on the right from the chair on the left? [In these, assume that the angle through which you view the chair conformations doesn't change.]
(a)
Problem 59e
For each pair of conformations shown, choose which is most stable. If both are equally stable, then write 'no difference.' [If both conformations have the same number of axial substituents, choose the one with the smallest axial substituents.]
(e)
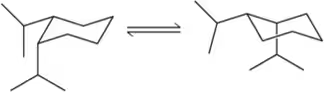
Problem 59g
For each pair of conformations shown, choose which is most stable. If both are equally stable, then write 'no difference.' [If both conformations have the same number of axial substituents, choose the one with the smallest axial substituents.]
(g)