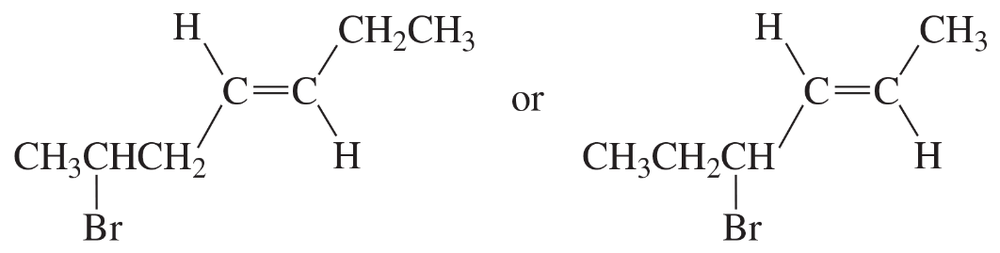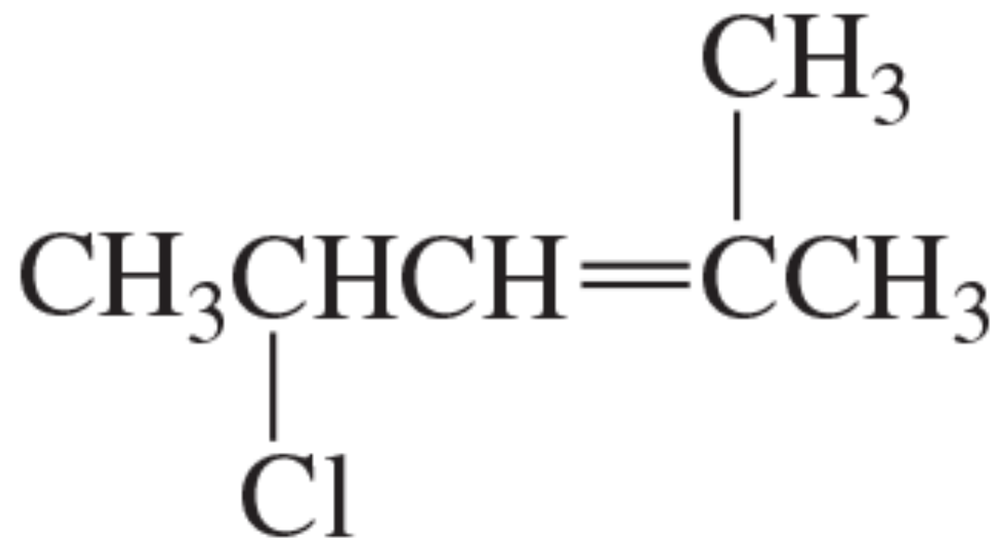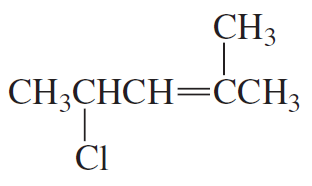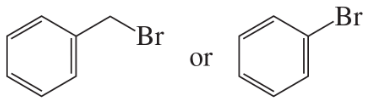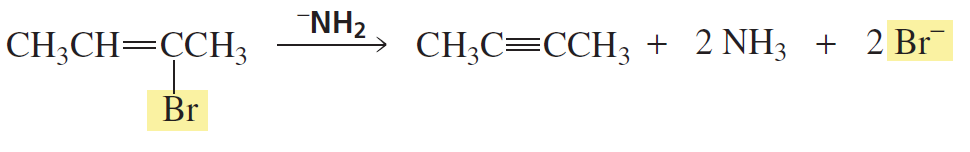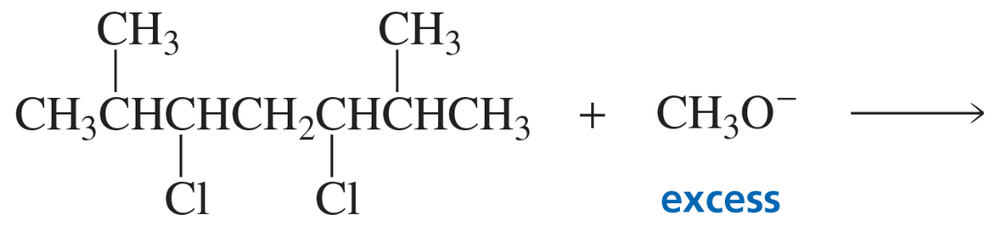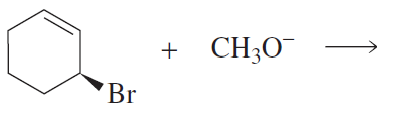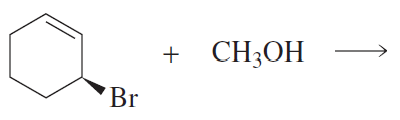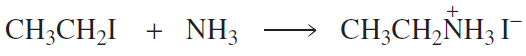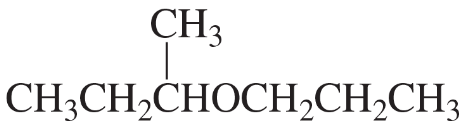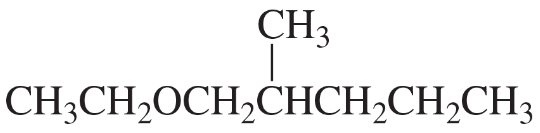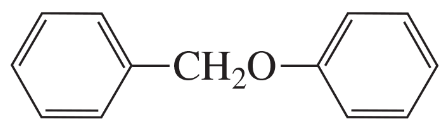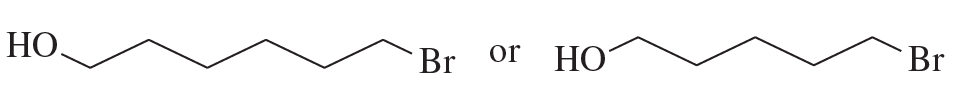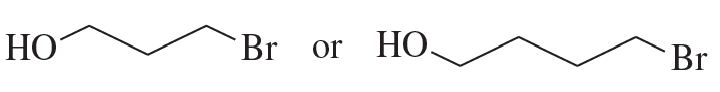Back
BackProblem 41
How does the ratio of substitution product to elimination product formed from the reaction of propyl bromide with CH3O− in methanol change if the nucleophile is changed to CH3S−?
Problem 42
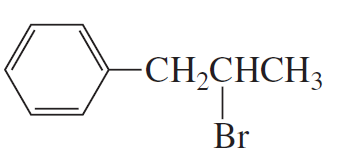
Explain why only a substitution product and no elimination product is obtained when the following compound reacts with sodium methoxide:
Problem 43a,b
a. Explain why 1-bromo-2,2-dimethylpropane has difficulty undergoing both SN2 and SN1 reactions.
b. Can it undergo E2 and E1 reactions?
Problem 44
a. Assuming that the two compounds shown below have the same stability, which one would you expect to be more reactive in an SN1 reaction?
b. Draw the products that each would form when the solvent is ethanol.
Problem 45a
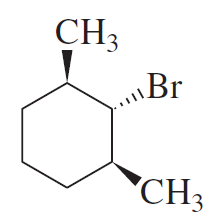
What products will be obtained from the E2 reaction of the following alkyl halides?
a.
Problem 45b
What products will be obtained from the E2 reaction of the following alkyl halides?
b.
Problem 45c
What products will be obtained from the E2 reaction of the following alkyl halides?
c.
Problem 46a
What products will be obtained from the E1 reaction of the alkyl halides in [PROBLEM 9-45]?
a.
Problem 46b
What products will be obtained from the E1 reaction of the alkyl halides in [PROBLEM 9-45]?
b.
Problem 46c
What products will be obtained from the E1 reaction of the alkyl halides in [PROBLEM 9-45]?
c.
Problem 47a
Draw the elimination products that are formed when 3-bromo-3-methyl-1-butene reacts with
a. CH3O−.
Problem 47b
Draw the elimination products that are formed when 3-bromo-3-methyl-1-butene reacts with
b. CH3OH
Problem 48
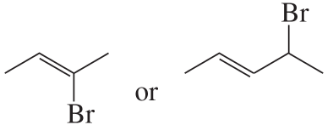
Which compound is more reactive in an SN1 reaction? In each case, you can assume that both alkyl halides have the same stability.
a.
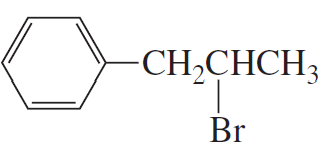
b.
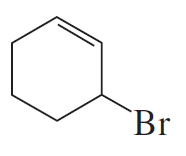
Problem 50
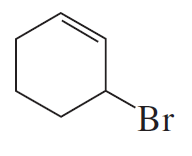
Why is a cumulated diene not formed in the reaction shown above?
Problem 52
What product is obtained when the following compound undergoes two successive elimination reactions?
Problem 53a
What products are formed from the following reactions?
a.
Problem 53b
What products are formed from the following reactions?
b.
Problem 54
Amines are good nucleophiles, even though they are neutral. How would the rate of an SN2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased?
Problem 55a
How will the rate of each of the following SN2 reactions change if it is carried out in a more polar solvent?
a.
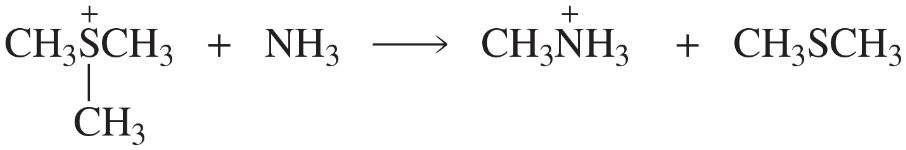
Problem 55b
How will the rate of each of the following SN2 reactions change if it is carried out in a more polar solvent?
b.
Problem 55c
How will the rate of each of the following SN2 reactions change if it is carried out in a more polar solvent?
c.
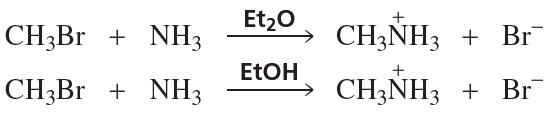
Problem 56a,b,c
Which reaction in each of the following pairs takes place more rapidly? (EtOH is ethyl alcohol; Et2O is diethyl ether.)
a.
b.
c.
Problem 56d,e
Which reaction in each of the following pairs takes place more rapidly? (EtOH is ethyl alcohol; Et2O is diethyl ether.)
d.
e.
Problem 58
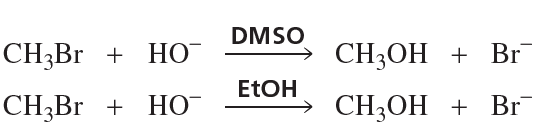
Would you expect acetate ion (CH3CO2−) to be a better nucleophile in an SN2 reaction with an alkyl halide carried out in methanol or in dimethyl sulfoxide?
Problem 59
Under which of the following reaction conditions will (R)-1-chloro-1-phenylethane form the most (R)-1-phenyl-1-ethanol: in water or in 1.0 M HO−?
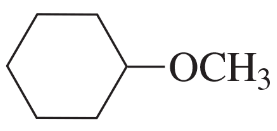
Problem 61a,b
What is the best way to prepare the following ethers using an alkyl halide and an alkoxide ion?
a.
b.
Problem 61c,d
What is the best way to prepare the following ethers using an alkyl halide and an alkoxide ion?
c.
d.
Problem 62
Identify the three products formed when 2-bromo-2-methylpropane is dissolved in a mixture of 80% ethanol and 20% water.
Problem 63b
What products (including stereoisomers, if applicable) are formed from the reaction of 3-bromo-3-methylpentane:
b. with H2O?
Problem 64a,b
After a proton is removed from the OH group, which compound in each pair forms a cyclic ether more rapidly?
a.
b.