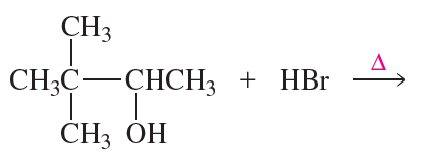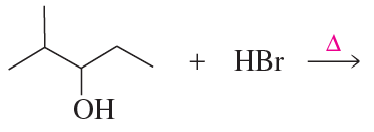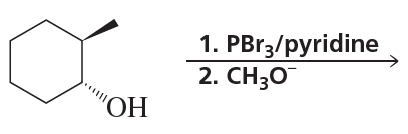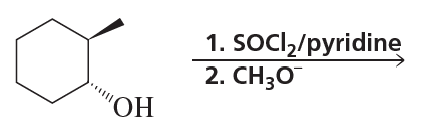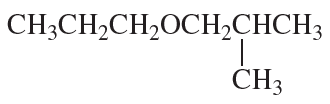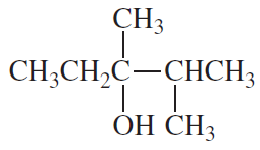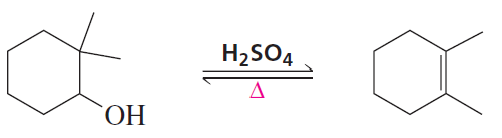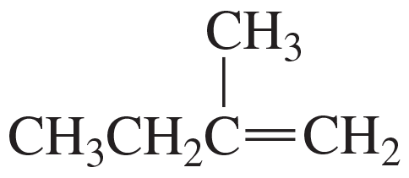Back
Back Bruice 8th Edition
Bruice 8th Edition Ch.10 - Reactions of Alcohols, Ethers, Epoxides, Amines, and Sulfur-Containing Compounds
Ch.10 - Reactions of Alcohols, Ethers, Epoxides, Amines, and Sulfur-Containing CompoundsProblem 1
Why are NH3 and CH3NH2 no longer nucleophiles when they are protonated?
Problem 3
Explain the difference in reactivity between CH3O+H2 and CH3OH in a nucleophilic substitution reaction. (The pKa of H3O+ is −1.7.)
Problem 5a
Explain how 1-butanol can be converted into the following compounds:
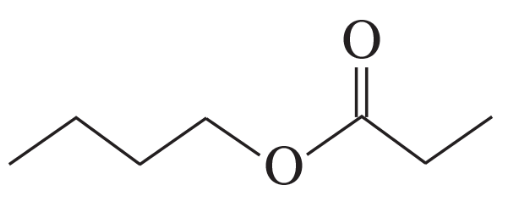
a.
Problem 5b
Explain how 1-butanol can be converted into the following compounds:
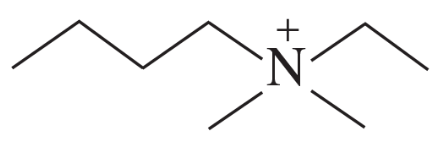
b.
Problem 6a
What is the major product of each of the following reactions?
a.
Problem 6b
What is the major product of each of the following reactions?
b.
Problem 6c
What is the major product of each of the following reactions?
c.
Problem 6d
What is the major product of each of the following reactions?
d.
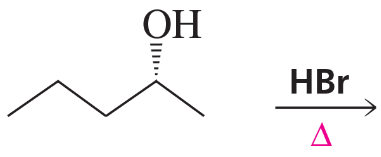
Problem 8a
What stereoisomers does the following reaction form?
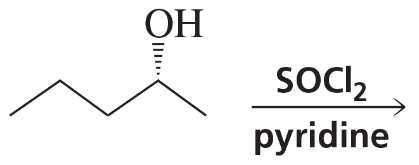
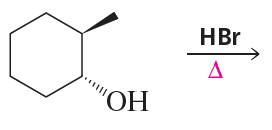
Problem 9a,b
What stereoisomers do the following reactions form?
a.
b.
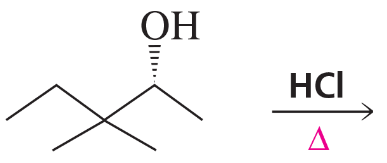
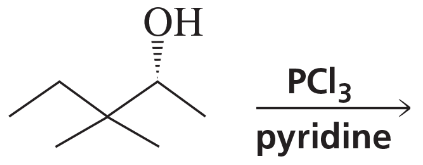
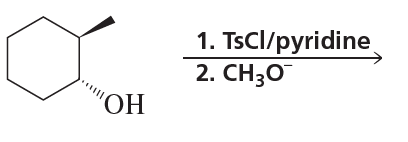
Problem 9c,d
What stereoisomers do the following reactions form?
c.
d.
- Fill in each box with the appropriate reagent: b.
Problem 10

Problem 11a
What stereoisomers do the following reactions form?
a.
Problem 11b
What stereoisomers do the following reactions form?
b.
Problem 11c
What stereoisomers do the following reactions form?
c.
Problem 11d
What stereoisomers do the following reactions form?
d.
Problem 12a
Show how 1-propanol can be converted into the following compounds by means of a sulfonate ester:
a. CH3CH2CH2SCH2CH3
Problem 12b
Show how 1-propanol can be converted into the following compounds by means of a sulfonate ester:
b.
Problem 13
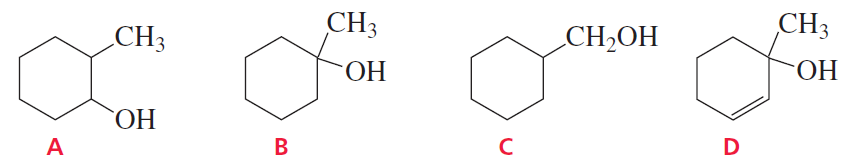
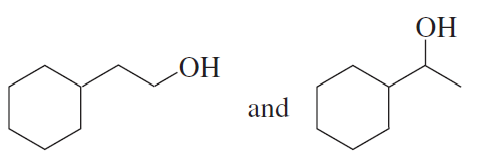
Which of the following alcohols dehydrates the fastest when heated with acid?
Problem 14(b)
What is the major product obtained when each of the following alcohols is heated in the presence of H2SO4?
b.
Problem 14a
What is the major product obtained when each of the following alcohols is heated in the presence of H2SO4?
a.
Problem 15a
Heating an alcohol with sulfuric acid is a good way to prepare a symmetrical ether such as diethyl ether.
a. Explain why it is not a good way to prepare an unsymmetrical ether such as ethyl propyl ether.
Problem 15b
Heating an alcohol with sulfuric acid is a good way to prepare a symmetrical ether such as diethyl ether.
b. How would you synthesize ethyl propyl ether?
Problem 16a
Propose a mechanism for each of the following reactions:
a.
Problem 18
Explain why the following alcohols, when heated with acid, form the same alkene.
Problem 19a
What stereoisomers are formed in the following reactions? Which stereoisomer is the major product?
a. the acid-catalyzed dehydration of 1-pentanol to 2-pentene
Problem 19b
What stereoisomers are formed in the following reactions? Which stereoisomer is the major product?
b. the acid-catalyzed dehydration of 3,4-dimethyl-3-hexanol to 3,4-dimethyl-3-hexene
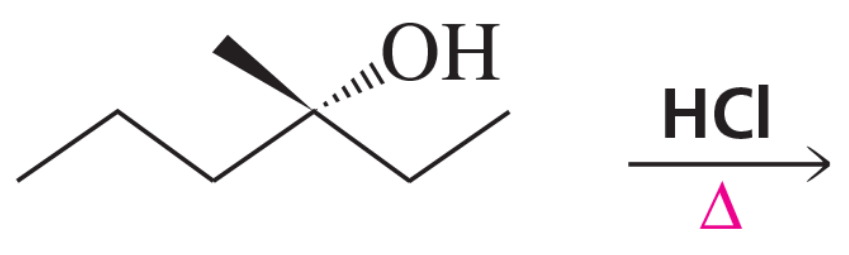
Problem 20
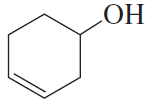
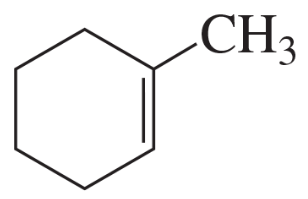
If the compound shown in the margin is heated in the presence of H2SO4,
a. what constitutional isomer would be formed in greatest yield?
b. what stereoisomer would be formed in greater yield?

Problem 21a,b
What alcohol would you treat with phosphorus oxychloride and pyridine to form each of the following alkenes?
a.
b.
Problem 22a(1,2,3)
What product is obtained from the reaction of each of the following alcohols with
a. H2CrO4?
1. 3-pentanol
2. 1-pentanol
3. 2-methyl-2-pentanol