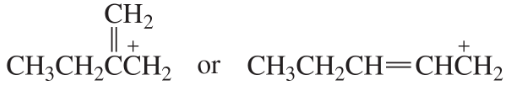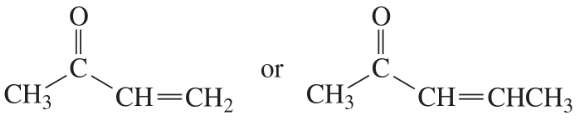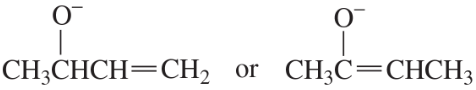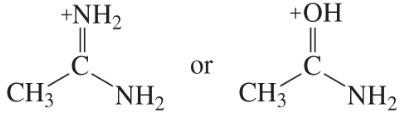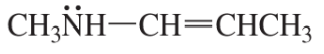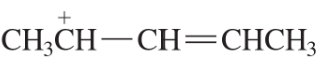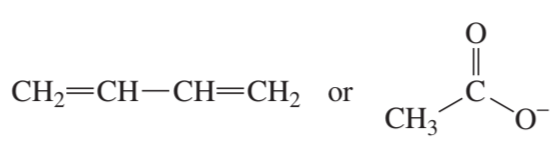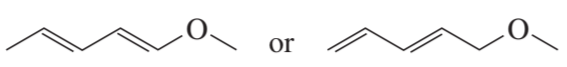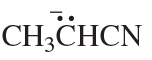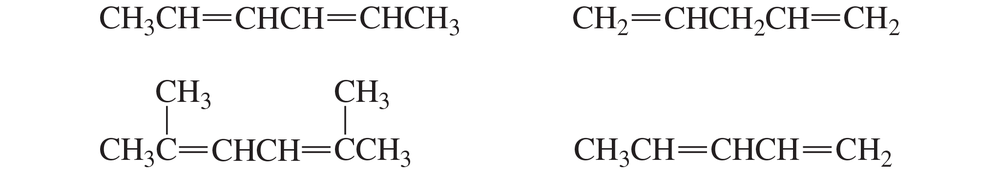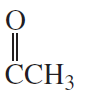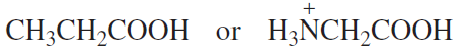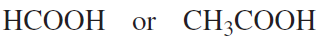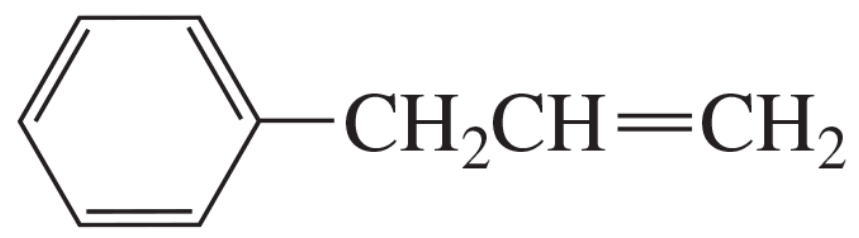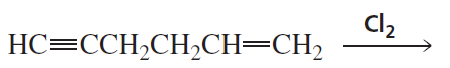Back
Back Bruice 8th Edition
Bruice 8th Edition Ch.8 - Delocalized Electrons:Their Effect on Stability, pKa, and the Products of a Reaction Aromaticity and Electronic Effects:An Introduction to the Reactions of Benzene
Ch.8 - Delocalized Electrons:Their Effect on Stability, pKa, and the Products of a Reaction Aromaticity and Electronic Effects:An Introduction to the Reactions of BenzeneProblem 1a
The following compounds have the same molecular formula as benzene. How many monobrominated products could each form?
1. HC≡CC≡CCH2CH3
2. CH2=CHC≡CCH=CH2
Problem 1c
How many dibrominated products could each of the compounds form if stereoisomers are included?
Problem 2
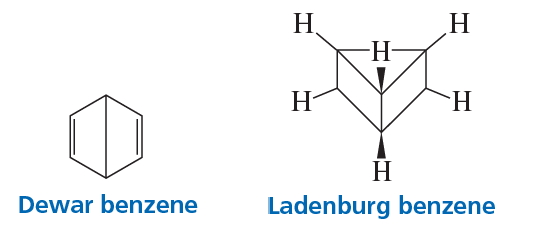
Between 1865 and 1890, other possible structures were proposed for benzene, such as those shown here. Considering what nineteenth-century chemists knew about benzene, which is a better proposal for benzene’s structure: Dewar benzene or Ladenburg benzene? Why?
Problem 4a
Which species in each pair is more stable?
a.
Problem 4b
Which species in each pair is more stable?
b.
Problem 4c
Which species in each pair is more stable?
c.
Problem 4d
Which species in each pair is more stable?
d.
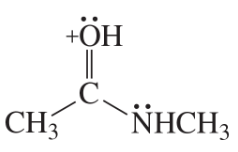
Problem 6b
Draw resonance contributors for each of the following species and rank them in order of decreasing contribution to the resonance hybrid. Then draw the resonance hybrid.
b.
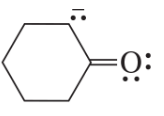
Problem 6c
Draw resonance contributors for each of the following species and rank them in order of decreasing contribution to the resonance hybrid. Then draw the resonance hybrid.
c.
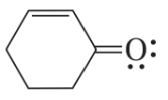
Problem 6d
Draw resonance contributors for each of the following species and rank them in order of decreasing contribution to the resonance hybrid. Then draw the resonance hybrid.
d.
Problem 6e
Draw resonance contributors for each of the following species and rank them in order of decreasing contribution to the resonance hybrid. Then draw the resonance hybrid.
e.
Problem 6f
Draw resonance contributors for each of the following species and rank them in order of decreasing contribution to the resonance hybrid. Then draw the resonance hybrid.
f.
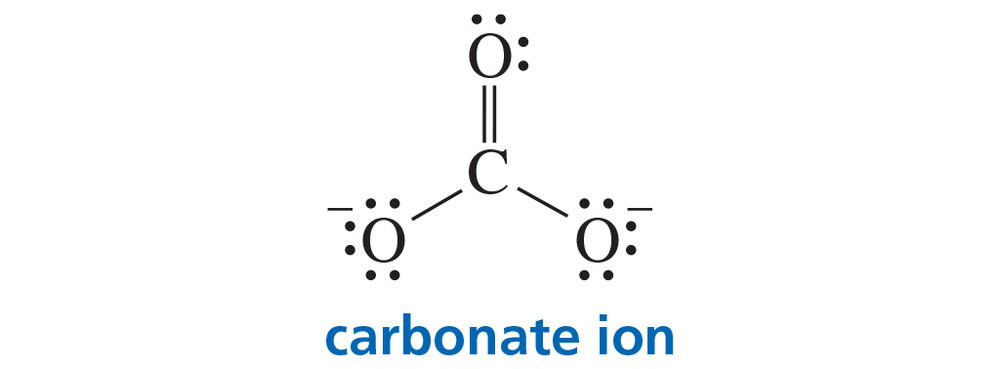
Problem 7
a. Predict the relative bond lengths of the three carbon–oxygen bonds in the carbonate ion.
b. What is the charge on each oxygen?
Problem 8a
List the following in order of decreasing delocalization energy:
Problem 9
Which has the greater delocalization energy?
Problem 10
Which has the greater delocalization energy?
Problem 11(8)
a. Draw resonance contributors for the following species. Do not include structures that are so unstable that their contributions to the resonance hybrid would be negligible. Indicate which are major contributors and which are minor contributors to the resonance hybrid.
b. Do any of the species have resonance contributors that all contribute equally to the resonance hybrid?
11.
Problem 12
Name the following dienes and rank them from most stable to least stable.
Problem 14
What is the total number of nodes in the 3 and 4 MOs of 1,3-butadiene?
Problem 15a,b,c
Answer the following questions for the MOs of 1,3-butadiene:
a. Which are bonding MOs, and which are * antibonding MOs?
b. Which MOs are symmetric, and which are antisymmetric?
c. Which MO is the HOMO and which is the LUMO in the ground state?
Problem 15d,e
Answer the following questions for the MOs of 1,3-butadiene:
d. Which MO is the HOMO and which is the LUMO in the excited state?
e. What is the relationship between the HOMO and the LUMO and symmetric and antisymmetric orbitals?
Problem 18a,b
Which member of each pair is the stronger base?
a. ethylamine or aniline
b. ethylamine or ethoxide ion
Problem 18c,d
Which member of each pair is the stronger base?
c. phenolate ion or ethoxide ion
d. phenolate ion or acetate ion
Problem 20a,b,c
For each of the following substituents, indicate whether it withdraws electrons inductively, donates electrons by hyperconjugation, withdraws electrons by resonance, or donates electrons by resonance.
a. Br
b. CH2CH3
c.
Problem 20d,e,f
For each of the following substituents, indicate whether it withdraws electrons inductively, donates electrons by hyperconjugation, withdraws electrons by resonance, or donates electrons by resonance.
d. NHCH3
e. OCH3
f. +N(CH3)3
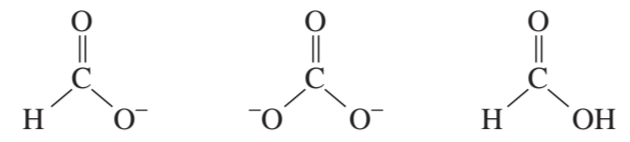
Problem 21c,d
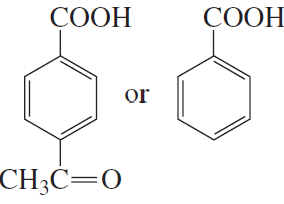
Which acid in each of the following pairs is stronger?
c.
d.
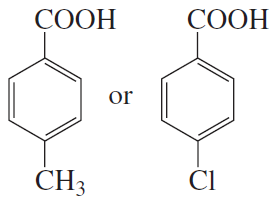
Problem 21e,f
Which acid in each of the following pairs is stronger?
e.
f.
Problem 23
Explain why the pKa of p-nitrophenol is 7.14, whereas the pKa of m-nitrophenol is 8.39.
Problem 24
What is the major product obtained from the addition of HBr to the following compound?
Problem 27b
What is the major product of each of the following reactions, assuming that one equivalent of each reagent is used in each reaction?
b.