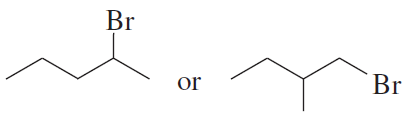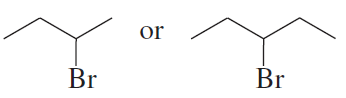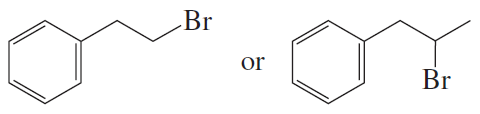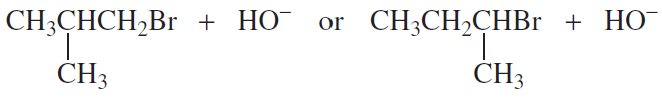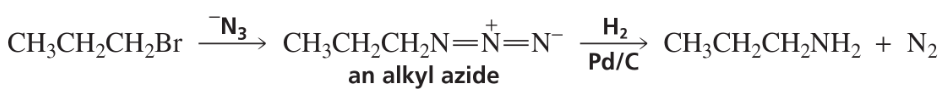Back
BackProblem 1
Draw the structure of DDE.
Problem 2
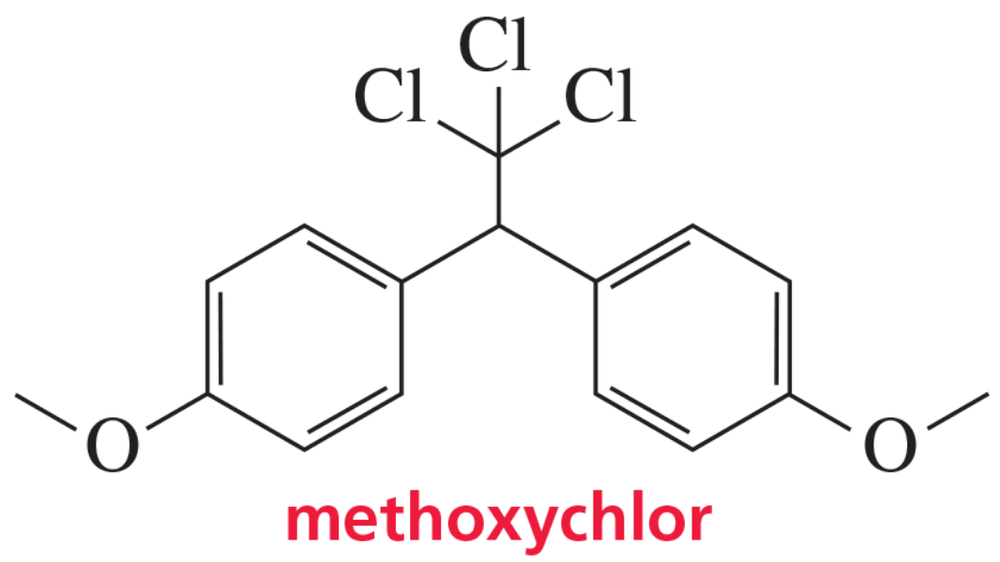
Methoxychlor is an insecticide that was intended to take DDT’s place because it is not as soluble in fatty tissues and is more readily biodegradable. It, too, can accumulate in the environment, however, so its use was also banned—in 2002 in the European Union and in 2003 in the United States. Why is methoxychlor less soluble in fatty tissues than DDT?
Problem 3a
How will the rate of the reaction between bromomethane and hydroxide ion be affected if the following changes in concentration are made?
a. The concentration of the alkyl halide is not changed and the concentration of the nucleophile is tripled.
Problem 3b
How will the rate of the reaction between bromomethane and hydroxide ion be affected if the following changes in concentration are made?
b. The concentration of the alkyl halide is cut in half and the concentration of the nucleophile is not changed.
Problem 3c
How will the rate of the reaction between bromomethane and hydroxide ion be affected if the following changes in concentration are made?
c. The concentration of the alkyl halide is cut in half and the concentration of the nucleophile is doubled.
Problem 4
Does increasing the energy barrier for an SN2 reaction increase or decrease the magnitude of the rate constant for the reaction?
Problem 5
Rank the following alkyl bromides from most reactive to least reactive in an SN2 reaction:
1-bromo-2-methylbutane, 1-bromo-3-methylbutane, 2-bromo-2-methylbutane, and 1-bromopentane.
Problem 6a
A small amount of another organic product is formed in a Williamson ether synthesis. What is this product when the alkyl halide used in the synthesis of butyl propyl ether is
a. propyl bromide?
Problem 6b
A small amount of another organic product is formed in a Williamson ether synthesis. What is this product when the alkyl halide used in the synthesis of butyl propyl ether is
b. butyl bromide?
Problem 6c,d
Draw the products obtained from the SN2 reaction of:
c. (S)-3-chlorohexane and hydroxide ion.
d. 3-iodopentane and hydroxide ion.
Problem 8
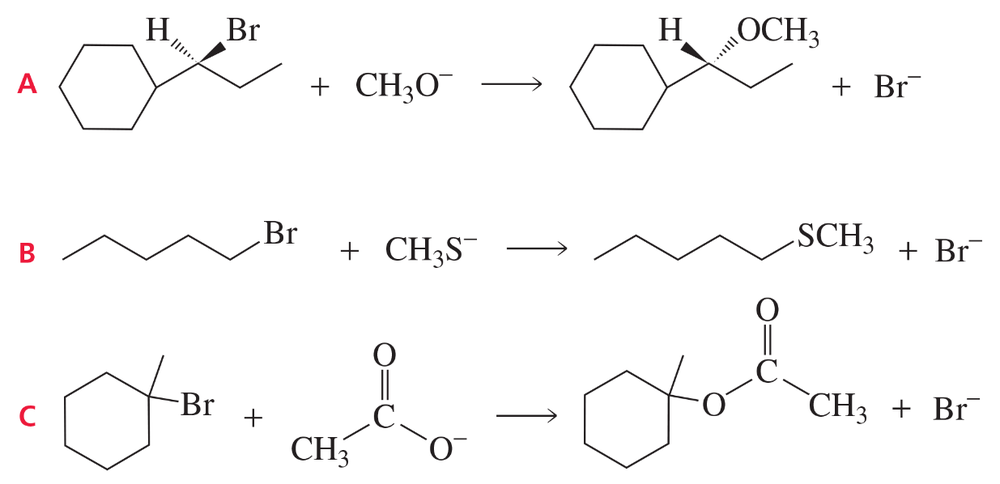
Draw the substitution product formed by each of the following SN2 reactions:
a. trans-1-iodo-4-ethylcyclohexane and methoxide ion
b. cis-1-chloro-3-methylcyclobutane and ethoxide ion
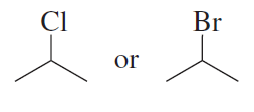
Problem 9a,b
Which alkyl halide is more reactive in an SN2 reaction with a given nucleophile?
a.
b.
Problem 9c,d
Which alkyl halide is more reactive in an SN2 reaction with a given nucleophile?
c.
d.
Problem 10a,b
Indicate whether each of the following solvents is protic or aprotic:
a. chloroform (CHCl3)
b. diethyl ether
Problem 10c,d
Indicate whether each of the following solvents is protic or aprotic:
c. acetic acid
d. hexane
Problem 11
a. Which is a stronger base: RO− or RS−?
b. Which is a better nucleophile in an aqueous solution?
c. Which is a better nucleophile in DMSO?
Problem 12c
Which is a better nucleophile?
c. CH3O− or CH3OH in H2O
Problem 12d
Which is a better nucleophile?
d. CH3O− or CH3OH in DMSO
Problem 12e,f
Which is a better nucleophile?
e. HO− or -NH2 in NH3
f. HO− or -NH2 in DMSO
Problem 14a
Which substitution reaction takes place more rapidly?
a. CH3CH2Br + H2O or CH3CH2Br + HO−
Problem 14b
Which substitution reaction takes place more rapidly?
b.
Problem 14c
Which substitution reaction takes place more rapidly?
c.
Problem 14d
Which substitution reaction takes place more rapidly?
d. CH3CH2Cl + I− or CH3CH2Br + I−
Problem 15a,b
What is the product of the reaction of bromoethane with each of the following nucleophiles?
a. CH3CH2CH2O−
b. CH3C≡C−
Problem 15c,d
What is the product of the reaction of bromoethane with each of the following nucleophiles?
c. (CH3)3N
d. CH3CH2S-
Problem 17a
Explain why the reaction of an alkyl halide with ammonia gives a low yield of primary amine.
Problem 17b
Explain why a much better yield of primary amine is obtained from the reaction of an alkyl halide with azide ion (-N3), followed by catalytic hydrogenation. (Hint: An alkyl azide is not nucleophilic.)
Problem 18a,b
Draw the stereoisomers that are formed from the following SN1 reactions:
a. 3-bromo-3-methylpentane and methanol
b. 3-chloro-3-methylhexane and methanol
Problem 19
Rank the following alkyl halides from most reactive to least reactive in an SN1 reaction:
2-bromo-2-methylpentane, 2-chloro-2-methylpentane, 3-chloropentane, and 2-iodo-2-methylpentane.
Problem 21a,b,c
Which of the following reactions take place more rapidly when the concentration of the nucleophile is increased?