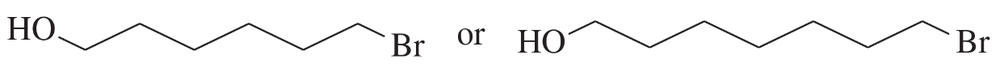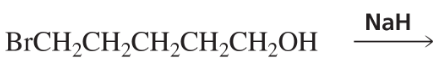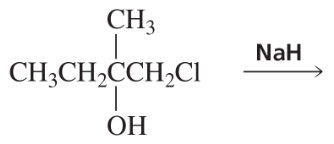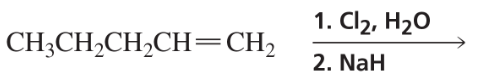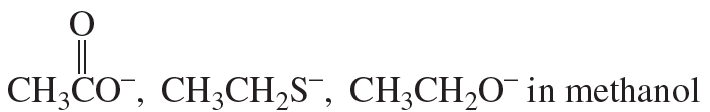Back
BackProblem 64c
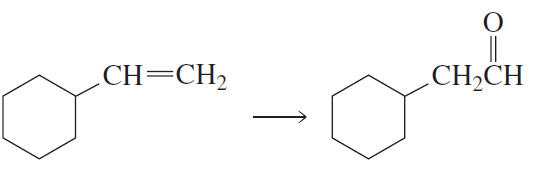
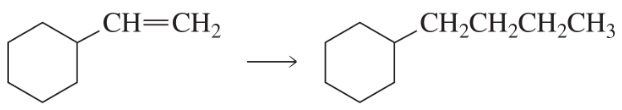
After a proton is removed from the OH group, which compound in each pair forms a cyclic ether more rapidly?
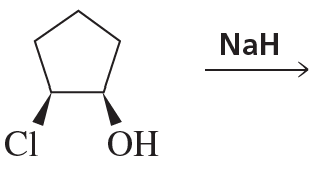
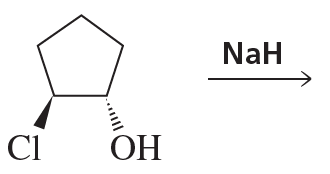
c.
Problem 65a,b
Draw the products of the following intramolecular reactions:
a.
b.
Problem 65c,d
Draw the products of the following intramolecular reactions:
c.
d.
Problem 65e
Draw the products of the following intramolecular reactions:
e.
Problem 66a
For each of the following target molecules, design a multistep synthesis to show how it can be prepared from the given starting material:
a.
Problem 66b
For each of the following target molecules, design a multistep synthesis to show how it can be prepared from the given starting material:
b.
Problem 66c
For each of the following target molecules, design a multistep synthesis to show how it can be prepared from the given starting material:
c.
Problem 66d
For each of the following target molecules, design a multistep synthesis to show how it can be prepared from the given starting material:
d.
Problem 67c
What product is formed when 1-bromopropane reacts with each of the following nucleophiles?
c. CH3S−
Problem 67d
What product is formed when 1-bromopropane reacts with each of the following nucleophiles?
d. HS−
Problem 68e,f
Which member of each pair is a better nucleophile in methanol?
e. I− or Br−
f. Cl− or Br−
Problem 69a,b
Which member in each pair in [PROBLEM 9-68] is a better leaving group?
a. H2O or HO−
b. NH3 or H2O
Problem 69c,d
Which member in each pair in [PROBLEM 9-68] is a better leaving group?
c. H2O or H2S
d. HO− or HS−
Problem 69e,f
Which member in each pair in [PROBLEM 9-68] is a better leaving group?
e. I− or Br−
f. Cl− or Br−
Problem 70a,b
What nucleophiles would form the following compounds as a result of reacting with 1-iodobutane?
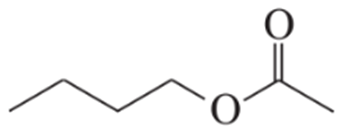
a.
b.
Problem 70e,f
What nucleophiles would form the following compounds as a result of reacting with 1-iodobutane?
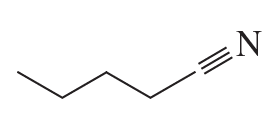
e.
f.
Problem 70g
What nucleophiles would form the following compounds as a result of reacting with 1-iodobutane?
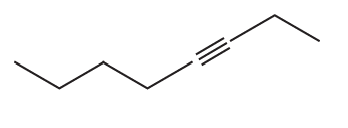
g.
Problem 70h
What nucleophiles would form the following compounds as a result of reacting with 1-iodobutane?
h.
Problem 71a,b
Explain how each of the following changes affect the rate of the reaction of 1-bromobutane with ethoxide ion in DMF.
a. The concentration of both the alkyl halide and the nucleophile are tripled.
b. The solvent is changed to ethanol.
Problem 71c,d
Explain how each of the following changes affect the rate of the reaction of 1-bromobutane with ethoxide ion in DMF.
c. The alkyl halide is changed to 1-chlorobutane.
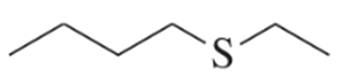
d. The alkyl halide is changed to 2-bromobutane.
Problem 72
Explain how the following changes affect the rate of the reaction of 2-bromo-2-methylbutane with methanol:
a. The alkyl halide is changed to 2-chloro-2-methylbutane.
b. The alkyl halide is changed to 2-chloro-3-methylbutane.
Problem 73a
Starting with cyclohexene, how can the following compounds be prepared?
a. methoxycyclohexane
Problem 73b
Starting with cyclohexene, how can the following compounds be prepared?
b. cyclohexylmethylamine
Problem 73c
Starting with cyclohexene, how can the following compounds be prepared?
c. dicyclohexyl ether
Problem 74a
Rank the following species in each set from best nucleophile to poorest nucleophile.
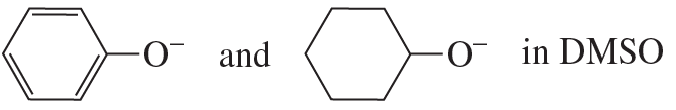
a.
Problem 74b
Rank the following species in each set from best nucleophile to poorest nucleophile.
b.
Problem 74c,d
Rank the following species in each set from best nucleophile to poorest nucleophile.
c. H2O and NH3 in methanol
d. Br−, Cl−, I− in methanol
Problem 75
The pKa of acetic acid in water is 4.76. What effect will a decrease in the polarity of the solvent have on the pKa? Why?
Problem 76
a. Identify the substitution products that form when 2-bromo-2-methylpropane is dissolved in a mixture of 80% ethanol and 20% water.
b. Explain why the same products are obtained when 2-chloro-2-methylpropane is dissolved in a mixture of 80% ethanol and 20% water.
Problem 77a,b
Draw the substitution products for each of the following reactions; if the products can exist as stereoisomers, show what stereoisomers are obtained:
a. (R)-2-bromopentane+CH3O−
b. (R)-3-bromo-3-methylheptane+CH3OH