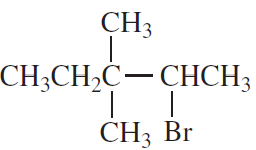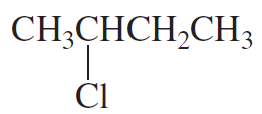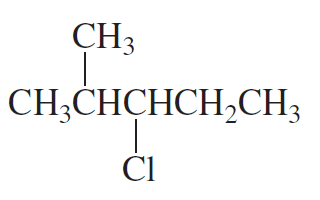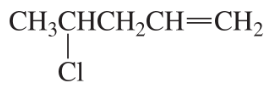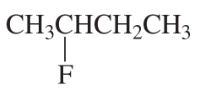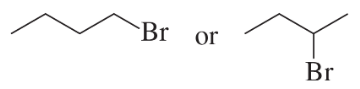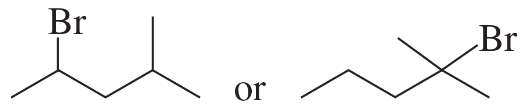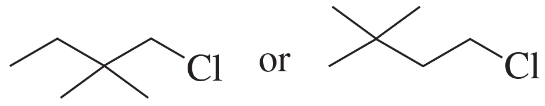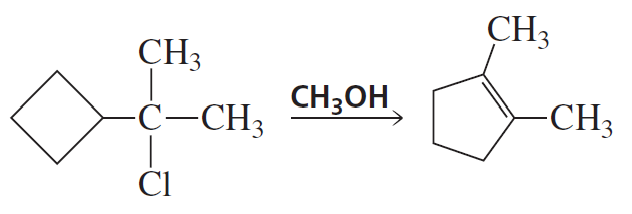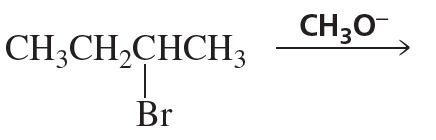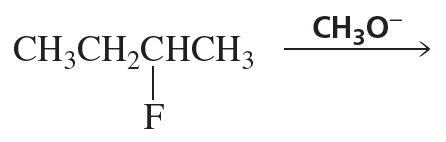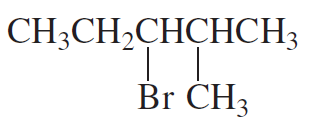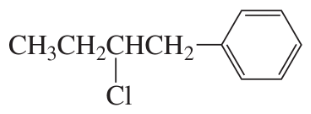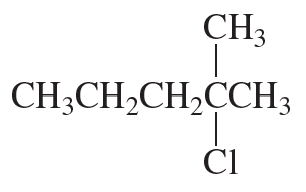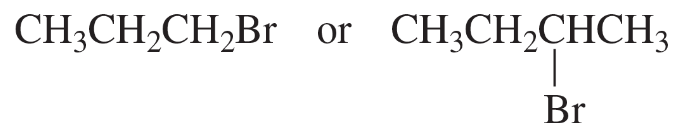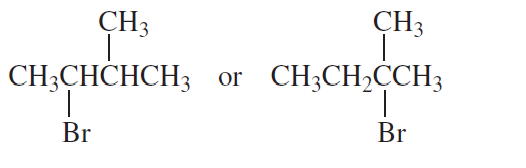Back
BackProblem 22a,b
What is the major elimination product obtained from the reaction of each of the following alkyl halides with hydroxide ion?
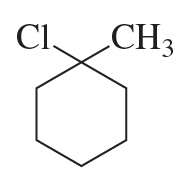
a.
b.
Problem 22c
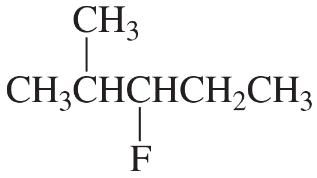
What is the major elimination product obtained from the reaction of each of the following alkyl halides with hydroxide ion?
c.
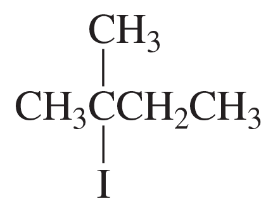
Problem 23a
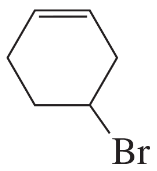
What is the major elimination product obtained from an E2 reaction of each of the following alkyl halides with hydroxide ion?
a.
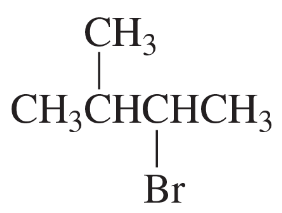
Problem 23b
What is the major elimination product obtained from an E2 reaction of each of the following alkyl halides with hydroxide ion?
b.
Problem 23c,d
What is the major elimination product obtained from an E2 reaction of each of the following alkyl halides with hydroxide ion?
c.
d.
Problem 23e
What is the major elimination product obtained from an E2 reaction of each of the following alkyl halides with hydroxide ion?
e.
Problem 23f
What is the major elimination product obtained from an E2 reaction of each of the following alkyl halides with hydroxide ion?
f.
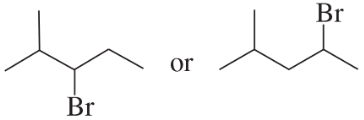
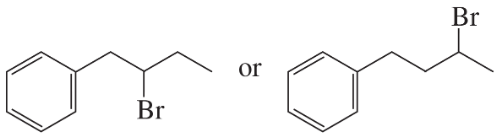
Problem 24a,b
Which alkyl halide in each pair is more reactive in an E2 reaction with hydroxide ion?
a.
b.
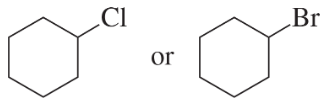
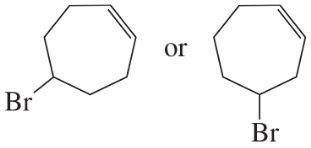
Problem 24c,d
Which alkyl halide in each pair is more reactive in an E2 reaction with hydroxide ion?
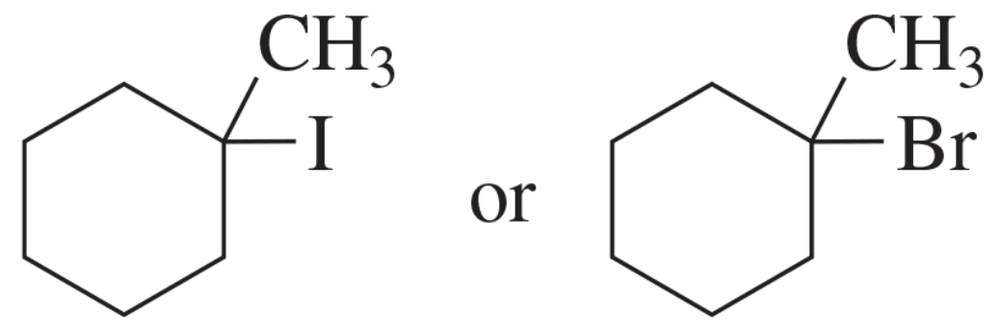
c.
d.
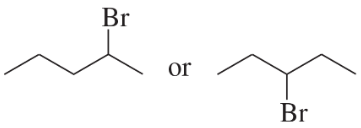
Problem 25a,b
Which alkyl halide in each pair is more reactive in an E2 reaction with hydroxide ion?
a.
b.
Problem 25c,d
Which alkyl halide in each pair is more reactive in an E2 reaction with hydroxide ion?
c.
d.
Problem 26
Four alkenes are formed from the E1 reaction of 3-bromo-2,3-dimethylpentane and methanol. Draw the structures of the alkenes and rank them according to the amount that would be formed.
Problem 27
If 2-fluoropentane could undergo an E1 reaction, would you expect the major product to be the more stable alkene or the less stable alkene? Explain your answer.
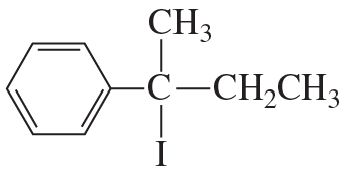
Problem 28a,b
Which of the following compounds would react faster in an
<IMAGE>
a. E1 reaction?
b. E2 reaction?
Problem 29
Propose a mechanism for the following reaction:
Problem 30a,b
For each of the following reactions, (1) decide whether an E2 or an E1 occurs, and (2) draw the major elimination product:
a.
b.
Problem 33(1)
a. What is the major product obtained when each of the following compounds undergoes an E2 reaction with methoxide ion? Show the configuration of the product.
b. Does the product obtained depend on whether you start with the R or S enantiomer of the reactant?
1.
Problem 33(3)
a. What is the major product obtained when each of the following compounds undergoes an E2 reaction with methoxide ion? Show the configuration of the product.
b. Does the product obtained depend on whether you start with the R or S enantiomer of the reactant?
3.
Problem 35a
What is the major product formed when the following compounds undergo an E1 reaction?
a.
Problem 35b
What is the major product formed when the following compounds undergo an E1 reaction?
b.
Problem 35c
What is the major product formed when the following compounds undergo an E1 reaction?
c.
Problem 36
Why do cis-1-bromo-2-ethylcyclohexane and trans-1-bromo-2-ethylcyclohexane form different major products when they undergo an E2 reaction?
Problem 37
Which isomer reacts more rapidly in an E2 reaction: cis-1-bromo-4-tert-butylcyclohexane or trans-1-bromo-4-tert-butylcyclohexane? Explain your answer.
Problem 38a
Draw the substitution and elimination products for the following reactions, showing the configuration of each product:
a. trans-1-chloro-2-methylcyclohexane + CH3O−
Problem 38b
Draw the substitution and elimination products for the following reactions, showing the configuration of each product:
b. cis-1-chloro-2-methylcyclohexane + CH3O−
Problem 38c,d
Draw the substitution and elimination products for the following reactions, showing the configuration of each product:
c. 1-chloro-1-methylcyclohexane + CH3O−
d. 1-chloro-1-methylcyclohexane + CH3OH
Problem 39a
Which reacts faster in an SN2 reaction?
Problem 39b
Which reacts faster in an E1 reaction?
Problem 39c
Which reacts faster in an SN1 reaction?
Problem 40
You were told in [SECTION 7.11] that is best to use a methyl halide or a primary alkyl halide for the reaction of an acetylide ion with an alkyl halide. Explain why this is so.