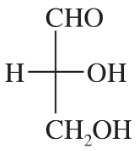
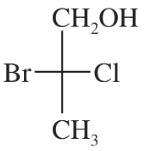
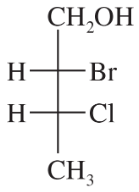
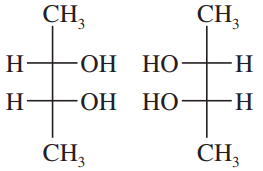
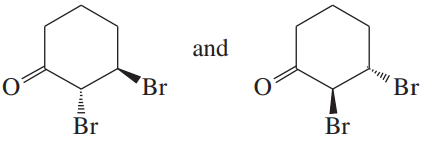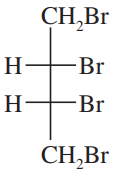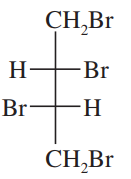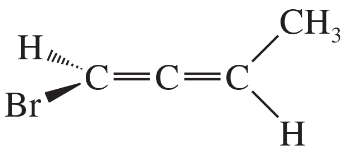Back
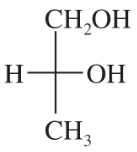
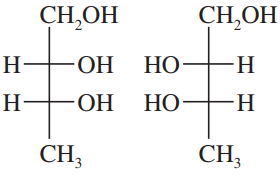
Back- Which configuration (R or S) does the bottom asymmetric carbon have for the D series of sugars? Which configuration for the L series?
Problem 23
- (a) Give the products expected when (+)@glyceraldehyde reacts with HCN. (b) What is the relationship between the products? How might they be separated? (c) Are the products optically active? Explain.
Problem 23
Problem 23a
Which of the following pairs of compounds could be separated by recrystallization or distillation?
a. meso-tartaric acid and (±)-tartaric acid (HOOC—CHOH—CHOH—COOH)
Problem 23b
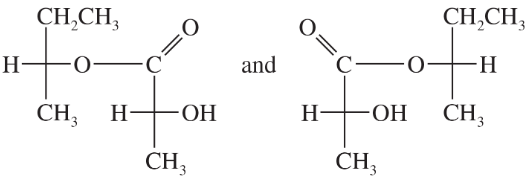
Which of the following pairs of compounds could be separated by recrystallization or distillation?
(b)
Problem 23c
Which of the following pairs of compounds could be separated by recrystallization or distillation?
(c)
Problem 24
To show that (R)-2-butyl (R,R)-tartrate and (S)-2-butyl (R,R)-tartrate are not enantiomers, draw and name the mirror images of these compounds.
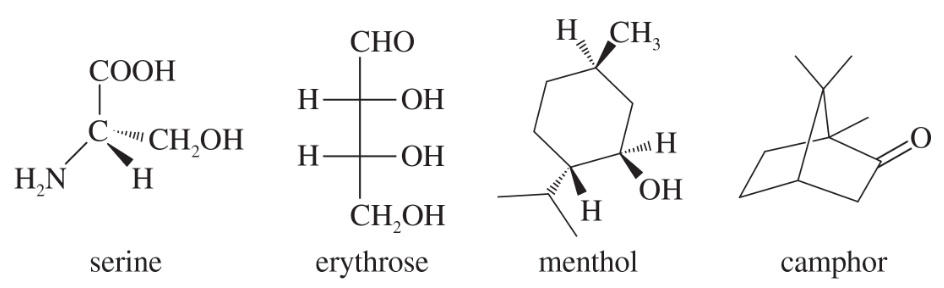
Problem 25
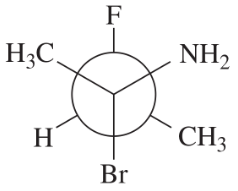
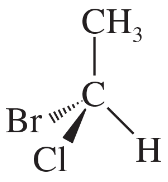
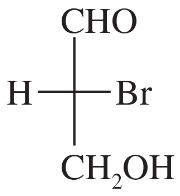
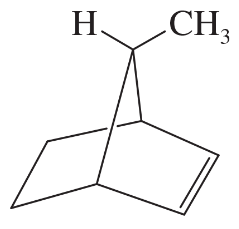
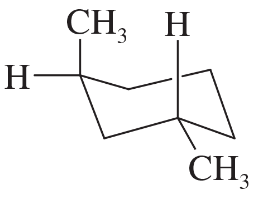
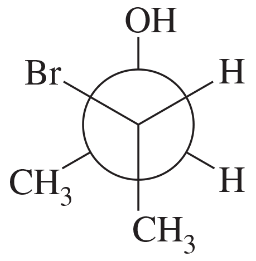
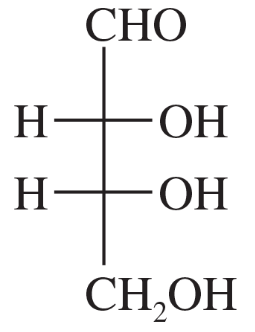
The following four structures are naturally occurring optically active compounds. Star (*) the asymmetric carbon atoms in these structures.
Problem 26a,b
For each structure,
1. star (*) any asymmetric carbon atoms.
2. label each asymmetric carbon as (R) or (S).
3. draw any internal mirror planes of symmetry.
4. label the structure as chiral or achiral.
5. label any meso structures.
(a)
(b)
Problem 26e
For each structure,
1. star (*) any asymmetric carbon atoms.
2. label each asymmetric carbon as (R) or (S).
3. draw any internal mirror planes of symmetry.
4. label the structure as chiral or achiral.
5. label any meso structures.
(e)
Problem 26f
For each structure,
1. star (*) any asymmetric carbon atoms.
2. label each asymmetric carbon as (R) or (S).
3. draw any internal mirror planes of symmetry.
4. label the structure as chiral or achiral.
5. label any meso structures.
(f)
Problem 26g,h
For each structure,
1. star (*) any asymmetric carbon atoms.
2. label each asymmetric carbon as (R) or (S).
3. draw any internal mirror planes of symmetry.
4. label the structure as chiral or achiral.
5. label any meso structures.
(g)
(h)
Problem 27a,b
For each of the compounds described by the following names,
1. draw a three-dimensional representation.
2. star (*) each chiral center.
3. draw any planes of symmetry.
4. draw any enantiomer.
5. draw any diastereomers.
6. label each structure you have drawn as chiral or achiral.
a. (S)-2-chlorobutane
b. (R)-1,1,2-trimethylcyclohexane
Problem 27c,d
For each of the compounds described by the following names,
1. draw a three-dimensional representation.
2. star (*) each chiral center.
3. draw any planes of symmetry.
4. draw any enantiomer.
5. draw any diastereomers.
6. label each structure you have drawn as chiral or achiral.
c. (2R,3S)-2,3-dibromohexane
d. (1R,2R)-1,2-dibromocyclohexane
Problem 28a,b
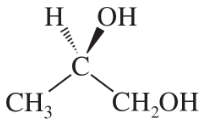
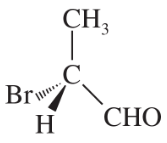
Convert the following perspective formulas to Fischer projections.
(a)
(b)
Problem 29a,b
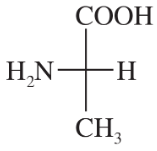
Convert the following Fischer projections to perspective formulas.
(a)
(b)
Problem 29c,d
Convert the following Fischer projections to perspective formulas
(c)
(d)
Problem 30a,b
Give the stereochemical relationships between each pair of structures. Examples are same compound, structural isomers, enantiomers, and diastereomers. Which pairs could you (theoretically) separate by distillation or recrystallization?
(a)
(b)
Problem 31a,b
Draw the enantiomer, if any, for each structure.
(a)
(b)
Problem 31e,f
Draw the enantiomer, if any, for each structure.
(e)
(f)
Problem 31g,h
Draw the enantiomer, if any, for each structure.
(g)
(h)
Problem 32a
Calculate the specific rotations of the following samples taken at 25 °C using the sodium D line.
a. 1.00 g of sample is dissolved in 20.0 mL of ethanol. Then 5.00 mL of this solution is placed in a 20.0-cm polarimeter tube. The observed rotation is 1.25° counterclockwise.
Problem 32b
Calculate the specific rotations of the following samples taken at 25 °C using the sodium D line.
b. 0.050 g of sample is dissolved in 2.0 mL of ethanol, and this solution is placed in a 2.0-cm polarimeter tube. The observed rotation is clockwise 0.043°.
Problem 33
(+)-Tartaric acid has a specific rotation Of +12.0°. Calculate the specific rotation of a mixture of 68% (+)-tartaric acid and 32% (–)-tartaric acid.
Problem 34
The specific rotation of (S)-2-iodobutane is +15.90°.
a. Draw the structure of (S)-2-iodobutane.
b. Predict the specific rotation of (R)-2-iodobutane.
c. Determine the percentage composition of a mixture of (R)- and (S)-2-iodobutane with a specific rotation of –7.95°.
Problem 35a
For each structure,
1. draw all the stereoisomers.
2. label each structure as chiral or achiral.
3. give the relationships between the stereoisomers (enantiomers, diastereomers).
(a)
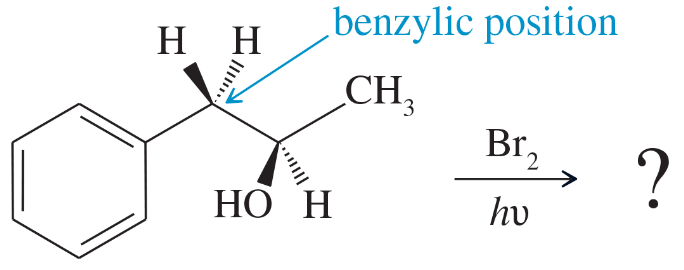
Problem 36a,b,c
Free-radical bromination of the following compound introduces bromine primarily at the benzylic position next to the aromatic ring. If the reaction stops at the monobromination stage, two stereoisomers result.
a. Propose a mechanism to show why free-radical halogenation occurs almost exclusively at the benzylic position.
b. Draw the two stereoisomers that result from monobromination at the benzylic position.
c. Assign R and S configurations to the asymmetric carbon atoms in the products.
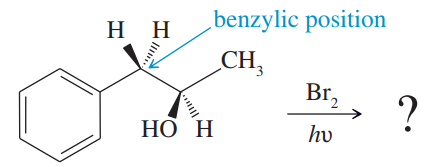
Problem 36d,e,f
Free-radical bromination of the following compound introduces bromine primarily at the benzylic position next to the aromatic ring. If the reaction stops at the monobromination stage, two stereoisomers result.
d. What is the relationship between the two isomeric products?
e. Will these two products be produced in identical amounts? That is, will the product mixture be exactly 50:50?
f. Will these two stereoisomers have identical physical properties such as boiling point, melting point, solubility, etc.? Could they be separated (theoretically, at least) by distillation or recrystallization?
Problem 38a,b
3,4-Dimethylpent-1-ene has the formula CH2=CH—CH(CH3)—CH(CH3)2. When pure (R)-3,4-dimethylpent-1-ene is treated with hydrogen over a platinum catalyst, the product is (S)-2,3-dimethylpentane.
a. Draw the equation for this reaction. Show the stereochemistry of the reactant and the product.
b. Has the chiral center retained its configuration during this hydrogenation, or has it been inverted?
Problem 38c
3,4-Dimethylpent-1-ene has the formula CH2=CH—CH(CH3)—CH(CH3)2. When pure (R)-3,4-dimethylpent-1-ene is treated with hydrogen over a platinum catalyst, the product is (S)-2,3-dimethylpentane.
c. The reactant is named (R), but the product is named (S). Does this name change imply a change in the spatial arrangement of the groups around the chiral center? So why does the name switch from (R) to (S)?
Problem 38d
3,4-Dimethylpent-1-ene has the formula CH2=CH—CH(CH3)—CH(CH3)2. When pure (R)-3,4-dimethylpent-1-ene is treated with hydrogen over a platinum catalyst, the product is (S)-2,3-dimethylpentane.
d. How useful is the (R) or (S) designation for predicting the sign of an optical rotation? Can you predict the sign of the rotation of the reactant? Of the product? (Hint from Juliet Capulet: “What’s in a name? That which we call a rose/By any other name would smell as sweet.”)