Back
BackProblem 1
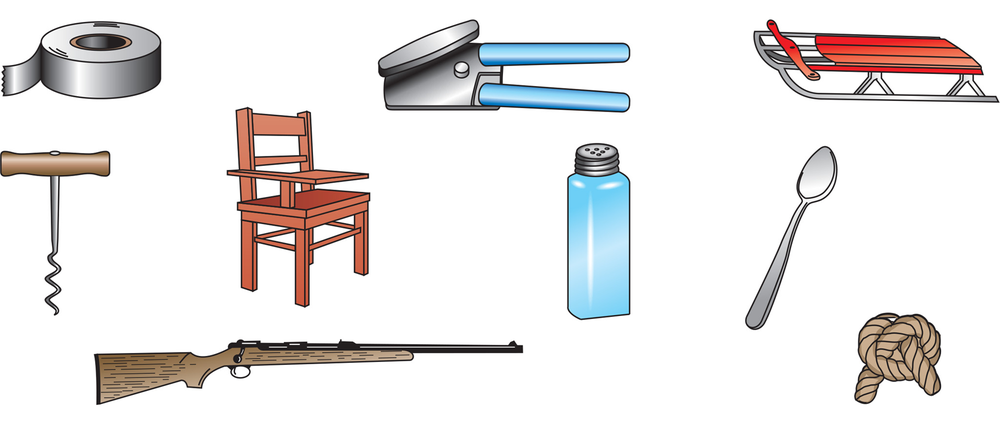
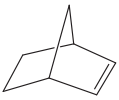
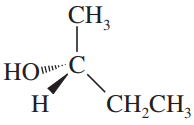
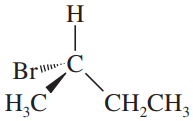
Determine whether the following objects are chiral or achiral.
Problem 2a,b
Make a model and draw a three-dimensional structure for each compound. Then draw the mirror image of your original structure and determine whether the mirror image is the same compound. Label each structure as being chiral or achiral, and label pairs of enantiomers.
(a) cis-1,2-dimethylcyclobutane
(b) trans-1,2-dimethylcyclobutane
Problem 2c,d
Make a model and draw a three-dimensional structure for each compound. Then draw the mirror image of your original structure and determine whether the mirror image is the same compound. Label each structure as being chiral or achiral, and label pairs of enantiomers.
(c) cis- and trans-1,3-dimethylcyclobutane
(d) 2-bromobutane
Problem 2e,f
Make a model and draw a three-dimensional structure for each compound. Then draw the mirror image of your original structure and determine whether the mirror image is the same compound. Label each structure as being chiral or achiral, and label pairs of enantiomers.
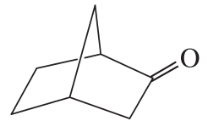
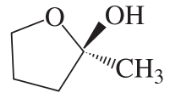
(e)
(f)
Problem 3a,b
Draw a three-dimensional structure for each compound, and star all asymmetric carbon atoms. Draw the mirror for each structure, and state whether you have drawn a pair of enantiomers or just the same molecule twice. Build molecular models of any of these examples that seem difficult to you
(a)
(b)
Problem 3c,d
Draw a three-dimensional structure for each compound, and star all asymmetric carbon atoms. Draw the mirror for each structure, and state whether you have drawn a pair of enantiomers or just the same molecule twice. Build molecular models of any of these examples that seem difficult to you.
(c)
(d) 1-bromo-2-methylbutane
Problem 3i
Draw a three-dimensional structure for each compound, and star all asymmetric carbon atoms. Draw the mirror for each structure, and state whether you have drawn a pair of enantiomers or just the same molecule twice. Build molecular models of any of these examples that seem difficult to you.
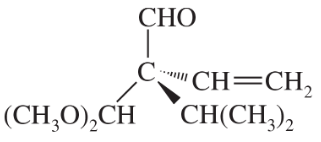
(i)
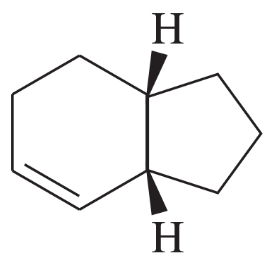
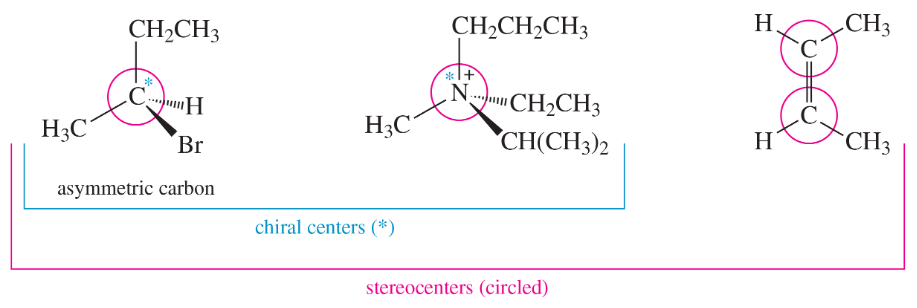
Problem 4a,b
For each of the stereocenters (circled) in Figure 5-5,
a. draw the compound with two of the groups on the stereocenter interchanged.
b. give the relationship of the new compound to the original compound.
- For each of the compounds described by the following names, 1. draw a three-dimensional representation. 2. star (*) each chiral center. 3. draw any planes of symmetry. 4. draw any enantiomer. 5. draw any diastereomers. 6. label each structure you have drawn as chiral or achiral. c. (2R,3S)-2,3-dibromohexane d. (1R,2R)-1,2-dibromocyclohexane
Problem 5
- Which of the following pairs of compounds could be separated by recrystallization or distillation? b.
Problem 5
and - Draw a three-dimensional structure for each compound, and star all asymmetric carbon atoms. Draw the mirror image for each structure, and state whether you have drawn a pair of enantiomers or just the same molecule twice. Build molecular models of any of these examples that seem difficult to you e. Chlorocyclohexane f. Cis-1,2-dichlorocyclobutane
Problem 5
- For each compound, determine whether the molecule has an internal mirror plane of symmetry. If it does, draw the mirror plane on a three-dimensional drawing of the molecule. If the molecule does not have an internal mirror plane, determine whether the structure is chiral. a. Methane b. cis-1,2-dibromocyclobutane c. trans-1,2-dibromocyclobutane
Problem 5
- Draw three-dimensional representations of the following compounds. Which have asymmetric carbon atoms? Which have no asymmetric carbons but are chiral anyway? Use your models for parts (a) through (d) and any others that seem unclear. c. ClHC═C═C(CH3)2 1-chloro-3-methylbuta-1,2-diene d. ClHC═CH―CH═CH2 1-chlorobuta-1,3-diene
Problem 5
- Make a model and draw a three-dimensional structure for each compound. Then draw the mirror image of your original structure and determine whether the mirror image is the same compound. Label each structure as being chiral or achiral, and label pairs of enantiomers. a. cis-1,2-dimethylcyclobutane b. trans-1,2-dimethylcyclobutane
Problem 5
- Draw three-dimensional representations of the following compounds. Which have asymmetric carbon atoms? Which have no asymmetric carbons but are chiral anyway? Use your models for parts (a) through (d) and any others that seem unclear. a. ClHC═C═CHCl 1,3-dichloropropadiene b. ClHC═C═CHCH3 1-chlorobuta-1,2-diene
Problem 5
- In Problem 5-3 , you drew the enantiomers for a number of chiral compounds. Now go back and designate each asymmetric carbon atom as either (R) or (S). e. Chlorocyclohexane f. Cis-1,2-dichlorocyclobutane
Problem 5
- To show that (R)-2-butyl (R,R)-tartrate and (S)-2-butyl (R,R)-tartrate are not enantiomers, draw and name the mirror images of these compounds.
Problem 5
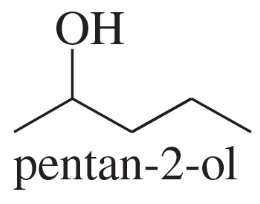
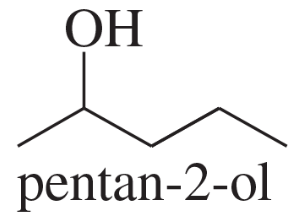
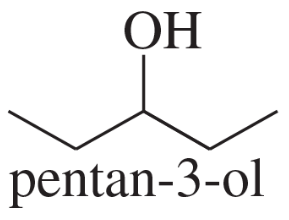
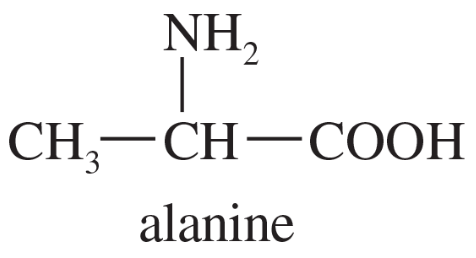
- Draw a Fischer projection for each compound. Remember that the cross represents an asymmetric carbon atom, and the carbon chain should be along the vertical, with the IUPAC numbering from top to bottom. c. (S)-1,2-dibromobutane d. (R)-butan-2-ol
Problem 5
- Draw a Fischer projection for each compound. Remember that the cross represents an asymmetric carbon atom, and the carbon chain should be along the vertical, with the IUPAC numbering from top to bottom. a. (S)-propane-1,2-diol b. (R)-2-bromobutan-1-ol
Problem 5
Problem 5a,b,c
For each compound, determine whether the molecule has an internal mirror plane of symmetry. If it does, draw the mirror plane on a three-dimensional drawing of the molecule. If the molecule does not have an internal mirror plane, determine whether the structure is chiral.
(a) methane
(b) cis-1,2-dibromocyclobutane
(c) trans-1,2-dibromocyclobutane
Problem 5d,e
For each compound, determine whether the molecule has an internal mirror plane of symmetry. If it does, draw the mirror plane on a three-dimensional drawing of the molecule. If the molecule does not have an internal mirror plane, determine whether the structure is chiral.
(d) 1,2-dichloropropane
(e)
Problem 5h
For each compound, determine whether the molecule has an internal mirror plane of symmetry. If it does, draw the mirror plane on a three-dimensional drawing of the molecule. If the molecule does not have an internal mirror plane, determine whether the structure is chiral.
(h)
- A solution of pure (S)-2-iodobutane ([α] = +15.90° ) in acetone is allowed to react with radioactive iodide, 131I-, until 1.0% of the iodobutane contains radioactive iodine. The specific rotation of this recovered iodobutane is found to be +15.58°. a. Determine the percentages of (R)- and (S)-2-iodobutane in the product mixture.
Problem 6
Problem 6a,b
Star (*) each asymmetric carbon atom in the following examples, and determine whether it has the (R) or (S) configuration.
(a)
(b)
Problem 6i
Star (*) each asymmetric carbon atom in the following examples, and determine whether it has the (R) or (S) configuration.
(i)
- b. Draw the six stereoisomers of octa-2,4,6-triene. Explain why there are only six stereoisomers, rather than the eight we might expect for a compound with three stereogenic double bonds.
Problem 7
Problem 7a,b
In Problem 5-3, you drew the enantiomers for a number of chiral compounds. Now go back and designate each asymmetric carbon atom as either (R) or (S).
(a)
(b)
Problem 7c,d
In Problem 5-3, you drew the enantiomers for a number of chiral compounds. Now go back and designate each asymmetric carbon atom as either (R) or (S).
(c)
(d) 1-bromo-2-methylbutane
Problem 7e,f
In Problem 5-3, you drew the enantiomers for a number of chiral compounds. Now go back and designate each asymmetric carbon atom as either (R) or (S).
(e) chlorocyclohexane
(f) cis-1,2-dichlorocyclobutane
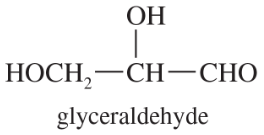
Problem 8a
A solution of 2.0 g of (+)-glyceraldehyde, HOCH2CHOHCHO, in 10.0 mL of water was placed in a 100-mm cell. Using the sodium D line, a rotation of +1.74° was found at 25 °C. Determine the specific rotation of (+)-glyceraldehyde