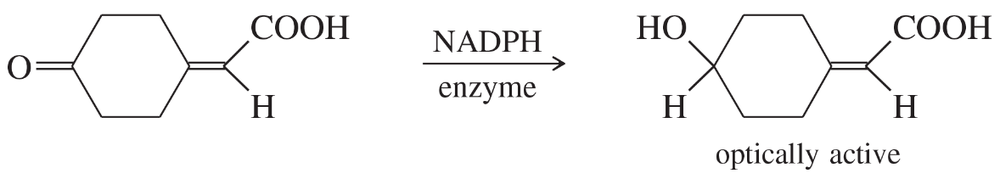Back
BackProblem 39c
A graduate student was studying enzymatic reductions of cyclohexanones when she encountered some interesting chemistry. When she used an enzyme and NADPH to reduce the following ketone, she was surprised to find that the product was optically active. She carefully repurified the product so that no enzyme, NADPH, or other contaminants were present. Still, the product was optically active.
c. If this reaction could be accomplished using H2 and a nickel catalyst, would the product be optically active? Explain.
Problem 41a
The original definition of meso is 'an achiral compound that has chiral diastereomers.' Our working definition of meso is 'an achiral compound that has chiral centers (usually asymmetric carbon atoms).' The working definition is much easier to apply, because we don't have to envision all possible chiral diastereomers of the compound. Still, the working definition is not quite as complete as the original definition.
a. Show how cis-cyclooctene is defined as a meso compound under the original definition, but not under our working definition. (Review Figure 5-19)
Problem 53e,f
Draw a three-dimensional structure for each compound, and star all asymmetric carbon atoms. Draw the mirror for each structure, and state whether you have drawn a pair of enantiomers or just the same molecule twice. Build molecular models of any of these examples that seem difficult to you.
(e) chlorocyclohexane
(f) cis-1,2-dichlorocyclobutane