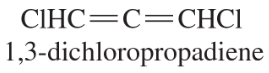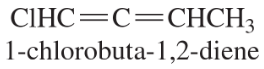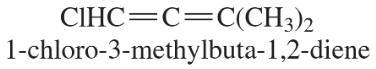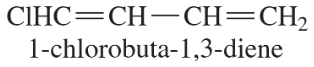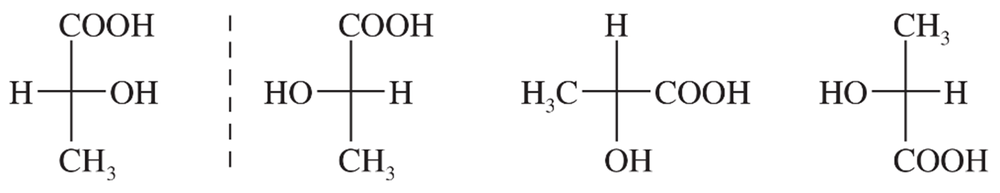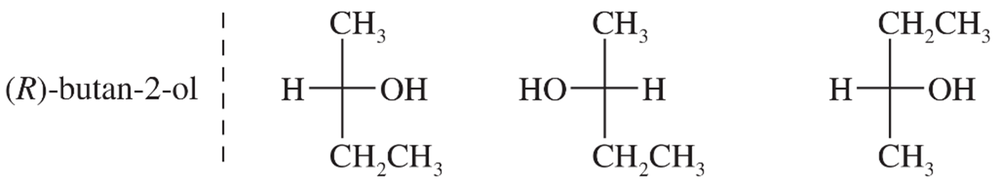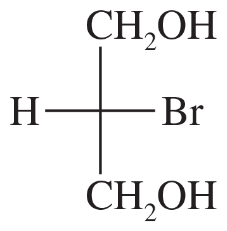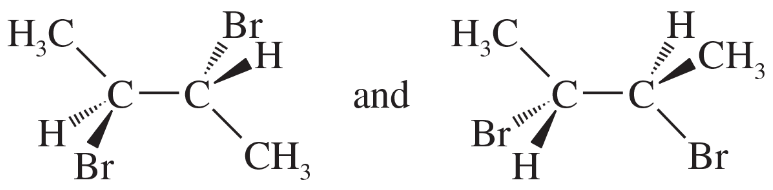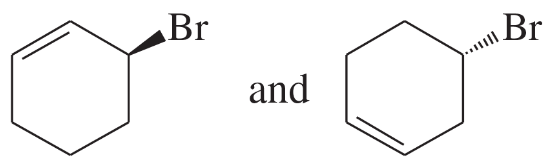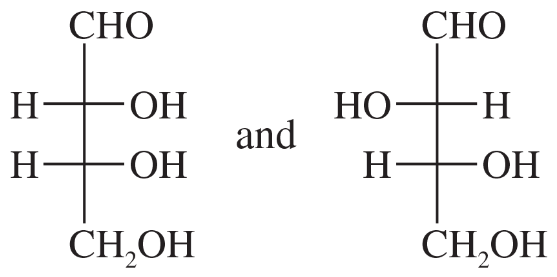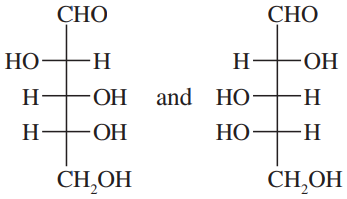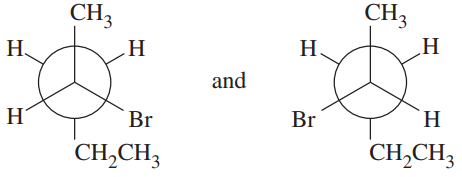Back
Back- Write structural formulas for the following compounds (includes both old- and new-style names). (j) vinylacetylene (k) (S)-3-methyl-1-penten-4-yne
Problem 9
Problem 9a
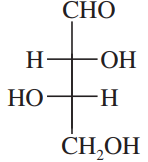
A solution of 0.50 g of (−)-epinephrine (see Figure 5-16) dissolved in 10.0 mL of dilute aqueous HCl was placed in a 20-cm polarimeter tube. Using the sodium D line, the rotation was found to be −5.1° at 25 °C. Determine the specific rotation of epinephrine.
<IMAGE>
Problem 10
A chiral sample gives a rotation that is close to 180°. How can one tell whether this rotation is +180° or -180°?
Problem 11
If you had the two enantiomers of carvone in unmarked bottles, could you use just your nose and a polarimeter to determine
a. whether it is the (+) or (−) enantiomer that smells like spearmint
b. whether it is the (R) or (S) enantiomer that smells like spearmint?
c. With the information given in the drawings of carvone above, what can you add to your answers to (a) and (b)?

Problem 12
When optically pure (R)-2-bromobutane is heated with water, butan-2-ol is the product. The reaction forms twice as much (S)-butan-2-ol as (R)-butan-2-ol. Calculate the e.e. and the specific rotation expected for the product.
Problem 13
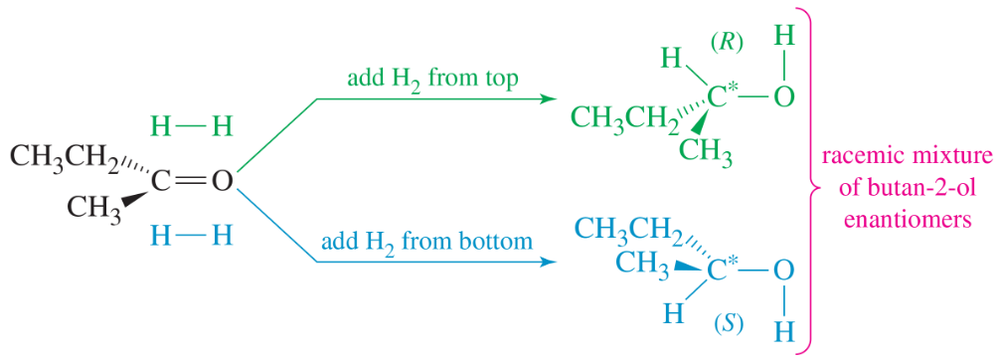
A chemist finds that the addition of (+)-epinephrine to the catalytic reduction of butan-2-one (Figure 5-17 ) gives a product that is slightly optically active, with a specific rotation of +0.45°. Calculate the percentages of (+)-butan-2-ol and (−)-butan-2-ol formed in this reaction.
- One of the crowning achievements of natural products synthesis was Bryostatin 1, published by Professor Gary Keck (University of Utah; Journal of the American Chemical Society, 2011, 133, 744–747). The Bryostatins are a family of compounds isolated from aquatic invertebrates known as Bryozoans. The compounds are of interest for a variety of biological effects, including anti-cancer activity and reversing brain damage in rodents. (d) How many chiral centers are in this molecule? (e) Using the number of chiral centers you reported in part (d), calculate the number of stereoisomers possible at these chiral centers. (Ignore stereoisomers at double bonds.)
Problem 14
Problem 14a,b
Make a model of each compound, draw it in its most symmetric conformation, and determine whether it is capable of showing optical activity.
a. 1-bromo-1-chloroethane
b. 1-bromo-2-chloroethane
Problem 15a,b
Draw three-dimensional representations of the following compounds. Which have asymmetric carbon atoms? Which have no asymmetric carbons but are chiral anyway? Use your models for parts (a) through (d) and any others that seem unclear.
(a)
(b)
Problem 15c,d
Draw three-dimensional representations of the following compounds. Which have asymmetric carbon atoms? Which have no asymmetric carbons but are chiral anyway? Use your models for parts (a) through (d) and any others that seem unclear.
(c)
(d)
Problem 15e,f
Draw three-dimensional representations of the following compounds. Which have asymmetric carbon atoms? Which have no asymmetric carbons but are chiral anyway? Use your models for parts (a) through (d) and any others that seem unclear.
(e)
(f)
Problem 15g
Draw three-dimensional representations of the following compounds. Which have asymmetric carbon atoms? Which have no asymmetric carbons but are chiral anyway? Use your models for parts (a) through (d) and any others that seem unclear.
(g)
Problem 16a
For each set of examples, make a model of the first structure, and indicate the relationship of each of the other structures to the first structure. Examples of relationships: same compound, enantiomer, structural isomer.
(a)
Problem 16c
For each set of examples, make a model of the first structure, and indicate the relationship of each of the other structures to the first structure. Examples of relationships: same compound, enantiomer, structural isomer.
(c)
Problem 17a,b
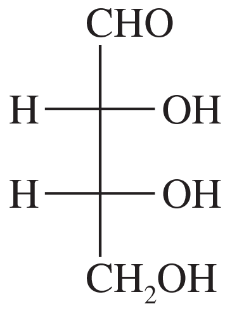
Draw a Fischer projection for each compound. Remember that the cross represents an asymmetric carbon atom, and the carbon chain should be along the vertical, with the IUPAC numbering from top to bottom.
(a) (S)-propane-1,2-diol
(b) (R)-2-bromobutan-1-ol
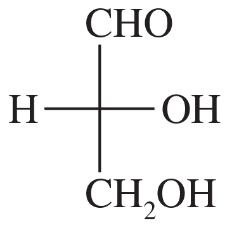
Problem 17c,d
Draw a Fischer projection for each compound. Remember that the cross represents an asymmetric carbon atom, and the carbon chain should be along the vertical, with the IUPAC numbering from top to bottom.
(c) (S)-1,2-dibromobutane
(d) (R)-butan-2-ol
Problem 17e
Draw a Fischer projection for each compound. Remember that the cross represents an asymmetric carbon atom, and the carbon chain should be along the vertical, with the IUPAC numbering from top to bottom.
(e)
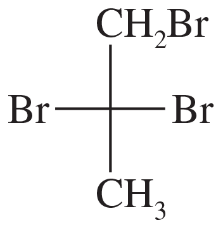
Problem 18c,d
For each Fischer projection,
1. make a model.
2. draw the mirror
3.. determine whether the mirror is the same as, or different from, the original structure.
4. draw any mirror planes of symmetry that are apparent from the Fischer projections.
(c)
(d)
Problem 19a,b
For each Fischer projection, label each asymmetric carbon atom as (R) or (S).
(a)
(b)
Problem 19c,d
For each Fischer projection, label each asymmetric carbon atom as (R) or (S).
(c)
(d)
Problem 19e,f
For each Fischer projection, label each asymmetric carbon atom as (R) or (S).
(e)
(f)
Problem 19g,h,i
For each Fischer projection, label each asymmetric carbon atom as (R) or (S).
(g)
(h)
(i)
Problem 20a,b
For each pair, give the relationship between the two compounds. Making models will be helpful.
(a) (2R,3S)-2,3-dibromohexane and (2S,3R)-2,3-dibromohexane
(b) (2R,3S)-2,3-dibromohexane and (2R,3R)-2,3-dibromohexane
Problem 20c,d
For each pair, give the relationship between the two compounds. Making models will be helpful.
(c)
(d)
Problem 20e,f
For each pair, give the relationship between the two compounds. Making models will be helpful.
(e)
(f)
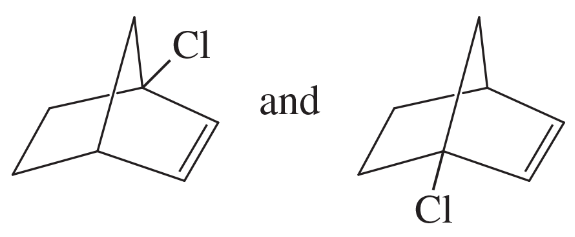
Problem 20g
For each pair, give the relationship between the two compounds. Making models will be helpful.
(g)
Problem 20h
For each pair, give the relationship between the two compounds. Making models will be helpful.
(h)
Problem 21c,d
Which of the following compounds are chiral? Draw each compound in its most symmetric conformation, star (*) any asymmetric carbon atoms, and draw any mirror planes. Label any meso compounds. You may use Fischer projections if you prefer.
(c) (2R,3S)-2-bromo-3-chlorobutane
(d) (2R,3S)-2,3-dibromobutane
Problem 21e,f
Which of the following compounds are chiral? Draw each compound in its most symmetric conformation, star (*) any asymmetric carbon atoms, and draw any mirror planes. Label any meso compounds. You may use Fischer projections if you prefer.
(e) (R,R)-2,3-dibromobutane
(f)
- Which configuration (R or S) does the bottom asymmetric carbon have for the D series of sugars? Which configuration for the L series?
Problem 23