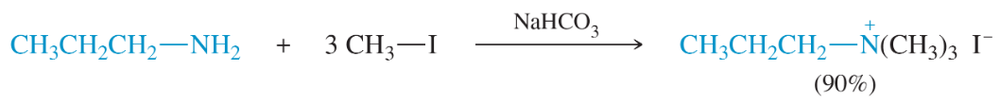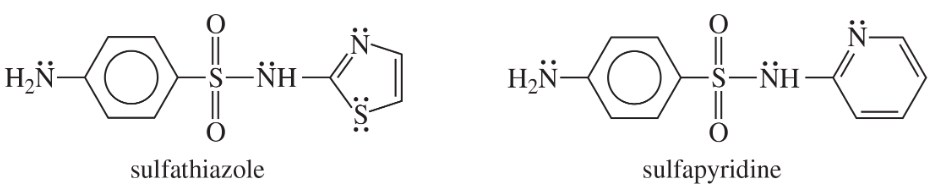Back
BackProblem 8
The proton and 13C NMR spectra of a compound of formula C4H11N are shown here. Determine the structure of this amine, and give peak assignments for all of the protons in the structure.
<IMAGE>
Problem 11
Propose a mechanism for nitration of pyridine at the 4-position, and show why this orientation is not observed.
Problem 12
Propose a mechanism for the sulfonation of pyridine, and point out why sulfonation occurs at the 3-position.
Problem 13
We have considered nucleophilic aromatic substitution of pyridine at the 2-position and 3-position but not at the 4-position. Complete the three possible cases by showing the mechanism for the reaction of methoxide ion with 4-chloropyridine. Show how the intermediate is stabilized by delocalization of the charge onto the nitrogen atom.
Problem 14a
(a) Propose a mechanism for the reaction of 2-bromopyridine with sodium amide to give 2-aminopyridine.
Problem 14b
(b) When 3-bromopyridine is used in this reaction, stronger reaction conditions are required and a mixture of 3-aminopyridine and 4-aminopyridine results. Propose a mechanism to explain this curious result.
Problem 15
Propose a mechanism to show the individual alkylations that form this quaternary ammonium salt.
Problem 16
Show how you would use direct alkylation to synthesize the following compounds.
(a) benzyltrimethylammonium iodide
(b) pentan-1-amine
(c) benzylamine
Problem 17
Give the products expected from the following reactions.
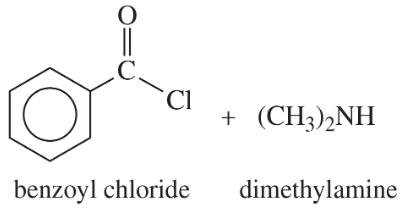
(a) acetyl chloride + ethylamine
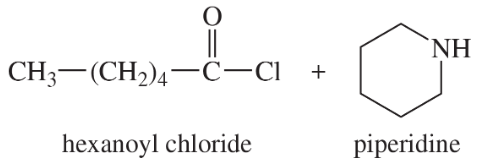
(b)
(c)
Problem 18
What would happen in the synthesis of sulfanilamide if the amino group were not protected as an amide in the chlorosulfonation step?
Problem 19
Show how you would use the same sulfonyl chloride as used in the sulfanilamide synthesis to make sulfathiazole and sulfapyridine.
Problem 19.39i
Predict the products of the following reactions:
(i) <IMAGE of reaction>
Problem 20a,b,c
Predict the major products formed when the following amines undergo exhaustive methylation, treatment with Ag2O, and heating.
(a) hexan-2-amine
(b) 2-methylpiperidine
(c) N-ethylpiperidine
Problem 20e
Predict the major products formed when the following amines undergo exhaustive methylation, treatment with Ag2O, and heating.
(e)
Problem 21a
Give the products expected when the following tertiary amines are treated with a peroxyacid and heated.
(a) N,N-dimethylhexan-2-amine
Problem 21b
Give the products expected when the following tertiary amines are treated with a peroxyacid and heated.
(b) N,N-diethylhexan-2-amine
Problem 21c
Give the products expected when the following tertiary amines are treated with a peroxyacid and heated.
(c) cyclohexyldimethylamine
Problem 21d
Give the products expected when the following tertiary amines are treated with a peroxyacid and heated.
(d) N-ethylpiperidine
Problem 22a
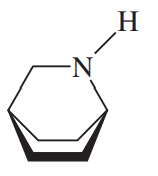
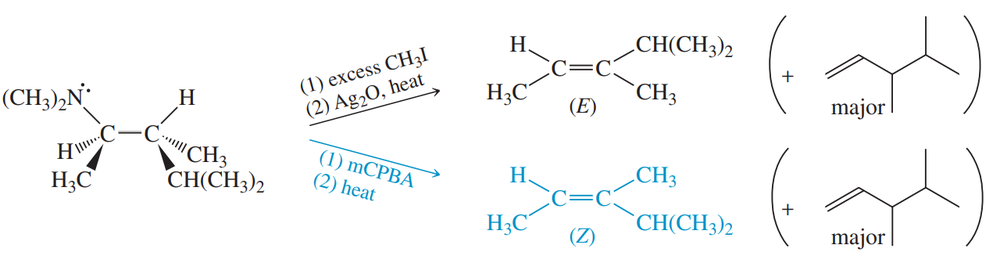
When the (R,R) isomer of the amine shown is treated with an excess of methyl iodide, then silver oxide, then heated, the major product is the Hofmann product.
(a) Draw the structure of the major (Hofmann) product.
Problem 22b
When the (R,R) isomer of the amine shown is treated with an excess of methyl iodide, then silver oxide, then heated, the major product is the Hofmann product.
(b) Some Zaitsev product is also formed. It has the (E) configuration. When the same amine is treated with mCPBA and heated, the Zaitsev product has the (Z) configuration. Use stereochemical drawings of the transition states to explain these observations.
Problem 23b
Predict the products from the reactions of the following amines with sodium nitrite in dilute HCl.
(b) N-ethylhexan-2-amine
Problem 23c
Predict the products from the reactions of the following amines with sodium nitrite in dilute HCl.
(c) piperidine
Problem 23d
Predict the products from the reactions of the following amines with sodium nitrite in dilute HCl.
(d) aniline
Problem 24
Propose a mechanism for the synthesis of methyl orange.
Problem 25a,b
Show how you would convert aniline to the following compounds.
(a) fluorobenzene
(b) chlorobenzene
Problem 25a,b,c
Give the expected products of lithium aluminum hydride reduction of the following compounds (followed by hydrolysis).
(a) butyronitrile
(b) N-cyclohexylacetamide
(c) ε-caprolactam
Problem 25c
Show how you would convert aniline to the following compounds.
(c) 1,3,5-trimethylbenzene
Problem 25d,e
Show how you would convert aniline to the following compounds.
(d) bromobenzene
(e) iodobenzene
Problem 25f
Show how you would convert aniline to the following compounds.
(f) benzonitrile
Problem 25g
Show how you would convert aniline to the following compounds.
(g) phenol