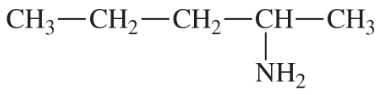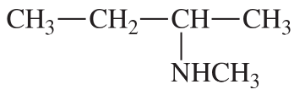Back
BackProblem 1
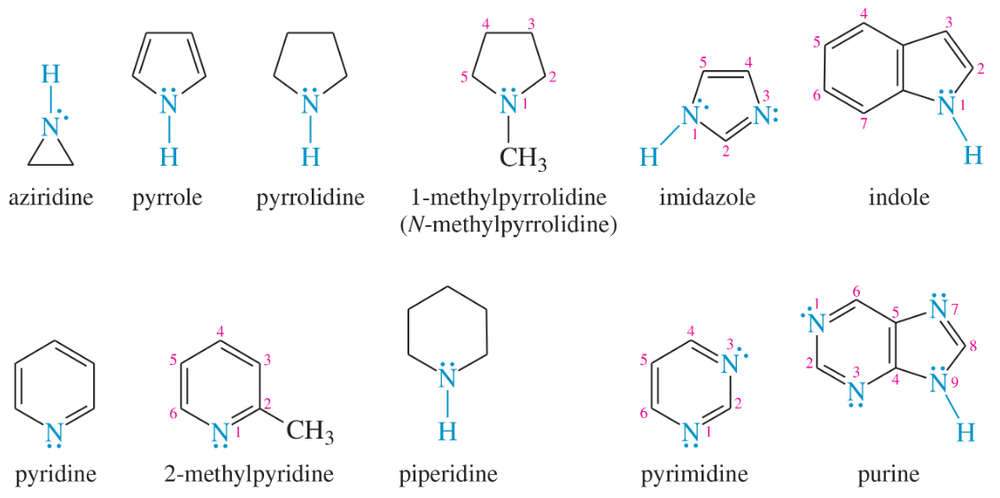
Determine which of the heterocyclic amines just shown are aromatic. Give the reasons for your conclusions.
Problem 2a,b,c
Draw the structures of the following compounds:
(a) tert-butylamine
(b) α-aminopropionaldehyde
(c) 4-(dimethylamino)pyridine
Problem 2d,e,f
Draw the structures of the following compounds:
(d) 2-methylaziridine
(e) N-ethyl-N-methylhexan-3-amine
(f) m-chloroaniline
Problem 3a,b,c
Give correct names for the following amines:
(a)
(b)
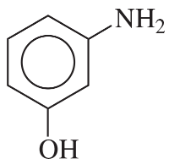
(c)
Problem 4
Which of the amines listed next is resolved into enantiomers? In each case, explain why interconversion of the enantiomers does or does not take place.
(a) cis-2-methylcyclohexanamine
(b) N-ethyl-N-methylcyclohexanamine
(c) N-methylaziridine
(d) ethylmethylanilinium iodide
(e) methylethylpropylisopropylammoniumiodide
Problem 5
Rank each set of compounds in order of increasing boiling points.
(a) triethylamine, di-n-propylamine, n-propyl ether
(b) ethanol, dimethylamine, dimethyl ether
(c) diethylamine, diisopropylamine, trimethylamine
Problem 6b
Rank each set of compounds in order of increasing basicity.
(b) aniline, p-methylaniline, p-nitroaniline
Problem 6c
Rank each set of compounds in order of increasing basicity.
(c) aniline, pyrrole, pyridine, piperidine
Problem 6d
Rank each set of compounds in order of increasing basicity.
(d) pyrrole, imidazole, 3-nitropyrrole