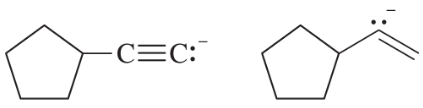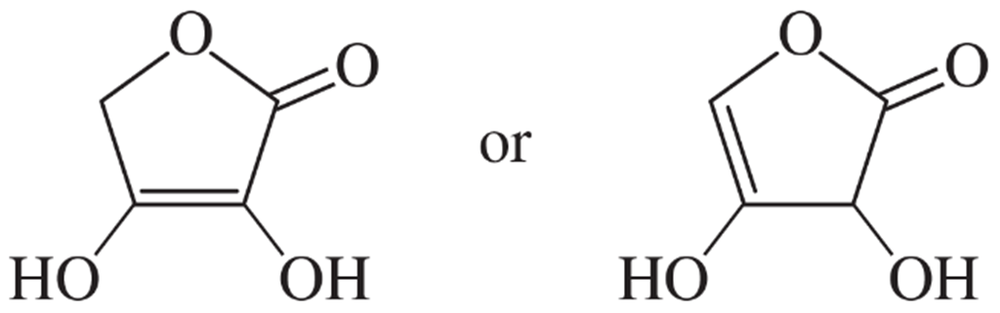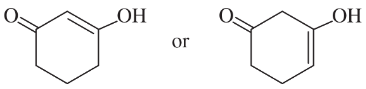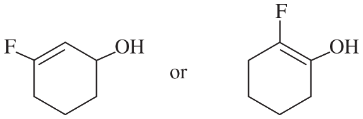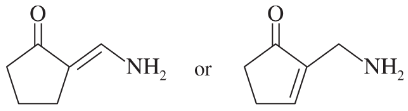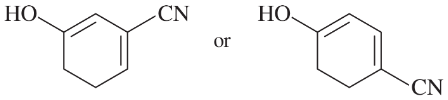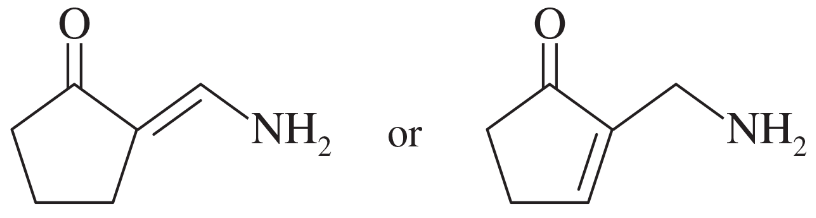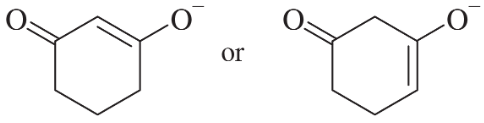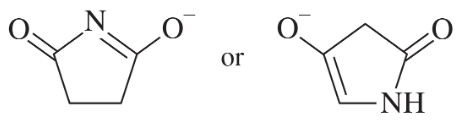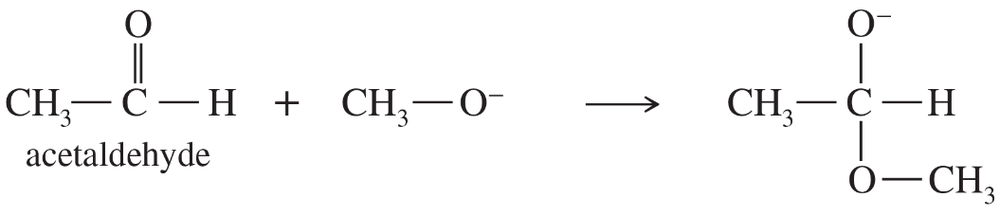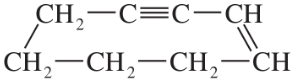Back
BackProblem 16b
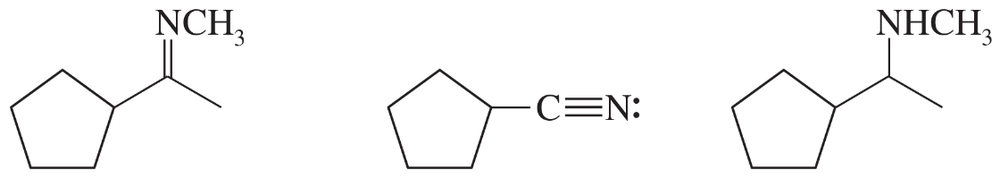
Give the structures of their conjugate acids, and estimate their pKas from similar compounds in Appendix 4.
Problem 17a,b
Consider each pair of bases, and explain which one is more basic. Draw their conjugate acids, and show which one is a stronger acid.
(a)
(b)
Problem 18
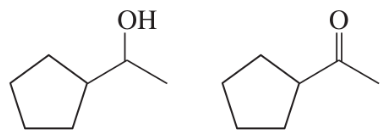
Which is a stronger base: ethoxide ion or acetate ion? Give pKb values (without looking them up) to support your choice.
Problem 20a
Acetic acid can also react as a very weak base (pKb = 20). Two different sites on acetic acid might become protonated to give the conjugate acid. Draw both of these possible conjugate acids, and explain (resonance) why the correct one is more stable. Calculate the pKa of this conjugate acid.
Problem 21a,b,c
Choose the more acidic member of each pair of isomers, and show why the acid you chose is more acidic.
(a)
(b)
(c)
Problem 21d,e
Choose the more acidic member of each pair of isomers, and show why the acid you chose is more acidic.
(d)
(e)
Problem 21f,g
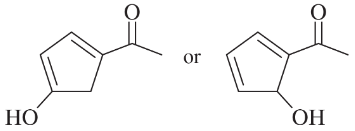
Choose the more acidic member of each pair of isomers, and show why the acid you chose is more acidic.
(f)
(g)
Problem 22a,b
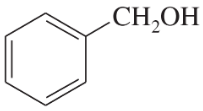
Choose the more basic member of each pair of isomers, and show why the base you chose is more basic.
(a)
(b)
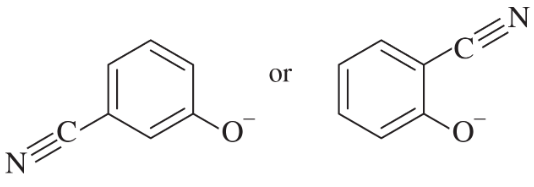
Problem 22c,d
Choose the more basic member of each pair of isomers, and show why the base you chose is more basic.
c.
d.
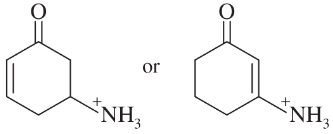
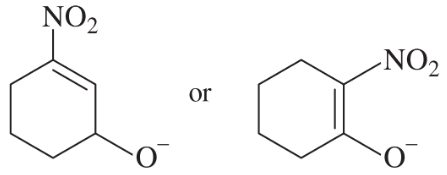
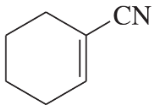
Problem 22e
Choose the more basic member of each pair of isomers, and show why the base you chose is more basic.
e.
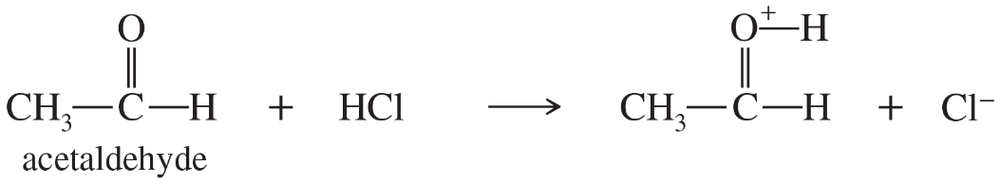
Problem 23a
In the following acid–base reactions,
1. draw Lewis structures of the reactants and the products.
2. determine which species are acting as electrophiles (acids) and which are acting as nucleophiles (bases).
3. use the curved-arrow formalism to show the movement of electron pairs in these reactions, as well as the imaginary movement in the resonance hybrids of the products.
4. indicate which reactions are best termed Brønsted–Lowry acid–base reactions.
(a)
Problem 23b
In the following acid–base reactions,
1. draw Lewis structures of the reactants and the products.
2. determine which species are acting as electrophiles (acids) and which are acting as nucleophiles (bases).
3. use the curved-arrow formalism to show the movement of electron pairs in these reactions, as well as the imaginary movement in the resonance hybrids of the products.
4. indicate which reactions are best termed Brønsted–Lowry acid–base reactions.
(b)
Problem 24a,b,c
Classify the following hydrocarbons, and draw a Lewis structure for each one. A compound may fit into more than one of the following classifications:
alkane
alkene
alkyne
cycloalkane
cycloalkene
cycloalkyne
aromatic
hydrocarbon
a. (CH3CH2)2CHCH(CH3)2
b. CH3CHCHCH2CH3
c.. CH3CCCH2CH2CH3
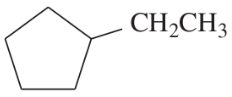
Problem 24d,e,f
Classify the following hydrocarbons, and draw a Lewis structure for each one. A compound may fit into more than one of the following classifications:
alkane
alkene
alkyne
cycloalkane
cycloalkene
cycloalkyne
aromatic
hydrocarbon
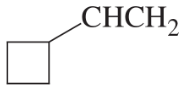
(d)
(e)
(f)
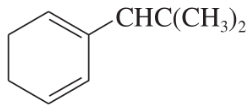
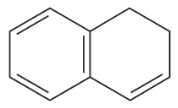
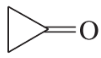
Problem 24g,h,i
Classify the following hydrocarbons, and draw a Lewis structure for each one. A compound may fit into more than one of the following classifications:
alkane
alkene
alkyne
cycloalkane
cycloalkene
cycloalkyne
aromatic
hydrocarbon
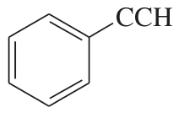
(g)
(h)
(i)
Problem 25a,b,c
Draw a Lewis structure, and classify each of the following compounds. The possible classifications are as follows:
alcohol
ether
ketone
aldehyde
carboxylic acid
alkene
(a) CH2CHCHO
(b) CH3CH2CH(OH)CH3
(c) CH3COCH2CH3
Problem 25d,e
Draw a Lewis structure, and classify each of the following compounds. The possible classifications are as follows: alcohol ether ketone aldehyde carboxylic acid alkene
(d) CH3CH2OCHCH2
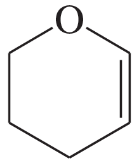
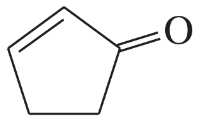
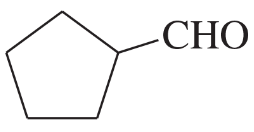
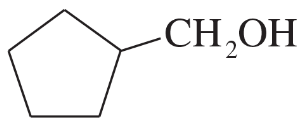
(e)
Problem 25f,g
Draw a Lewis structure, and classify each of the following compounds. The possible classifications are as follows:
alcohol
ether
ketone
aldehyde
carboxylic acid
alkene
(f)
(g)
Problem 25h,i
Draw a Lewis structure, and classify each of the following compounds. The possible classifications are as follows:
alcohol
ether
ketone
aldehyde
carboxylic acid
alkene
(h)
(i)
Problem 26a,b
Draw a Lewis structure, and classify each of the following compounds:
(a) CH3CH2CONHCH3
(b) (CH3CH2)2NH
Problem 26c,d
Draw a Lewis structure, and classify each of the following compounds:
(c) (CH3)2CHCOOCH3
(d) CH3CHCHCOCl
Problem 26e,f
Draw a Lewis structure, and classify each of the following compounds:
(e) (CH3CH2)2O
(f) CH3CH2CH2CN
Problem 26g,h
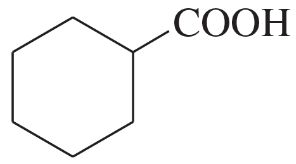
Draw a Lewis structure, and classify each of the following compounds:
(g) (CH3)3CCH2CH2COOH
(h)
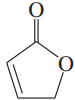
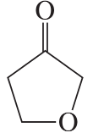
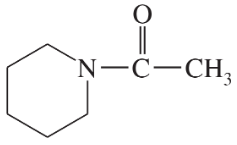
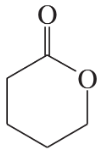
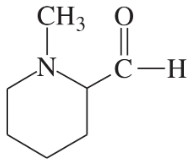
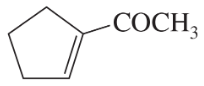
Problem 26i,j
Draw a Lewis structure, and classify each of the following compounds:
(i)
(j)
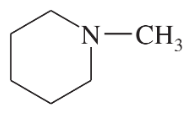
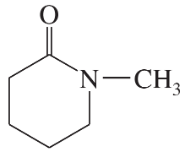
Problem 26k,l
Draw a Lewis structure, and classify each of the following compounds:
(k)
(l)
Problem 26m,n,o
Draw a Lewis structure, and classify each of the following compounds:
(m)
(n)
(o)
Problem 27a,b,c
Circle the functional groups in the following structures. State to which class (or classes) of compounds the structure belongs.
a. CH2=CHCH2COOCH3
b. CH3OCH3
c. CH3CHO
Problem 27d,e,f
Circle the functional groups in the following structures. State to which class (or classes) of compounds the structure belongs.
d. CH3CONH2
e. CH3NHCH3
f. RCOOH
Problem 27g,h,i
Circle the functional groups in the following structures. State to which class (or classes) of compounds the structure belongs.
(g)
(h)
(i)
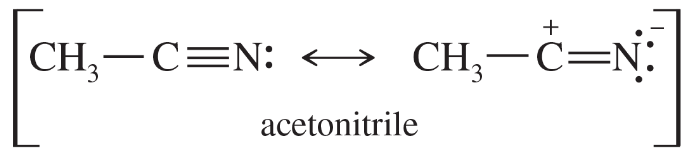
Problem 28
The C≡N triple bond in acetonitrile has a dipole moment of about 3.6 D and a bond length of about 1.16 Å. Calculate the amount of charge separation in this bond. How important is the charge-separated resonance form in the structure of acetonitrile?