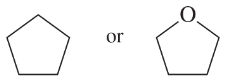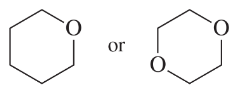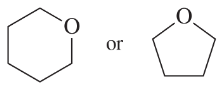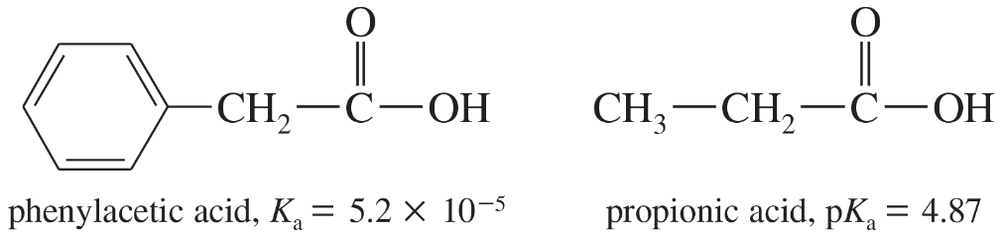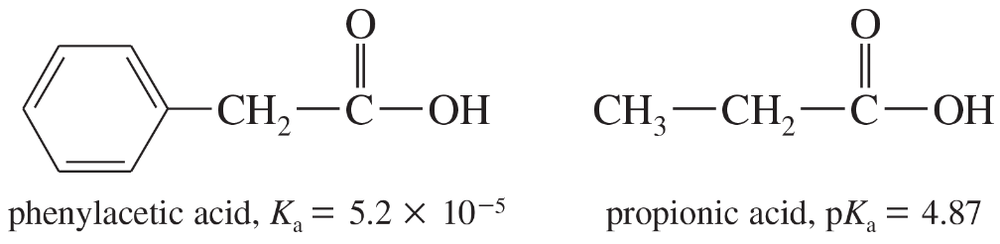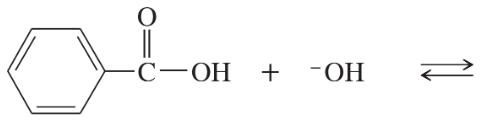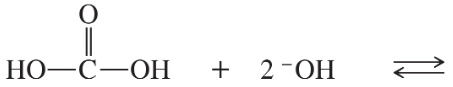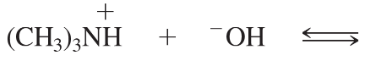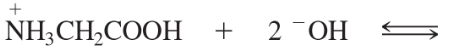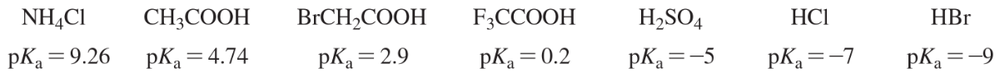Back
BackProblem 31e-h
Which of the following pure compounds can form hydrogen bonds? Which can form hydrogen bonds with water? Which ones do you expect to be soluble in water?
e. CH3(CH2)3CH3
f. CH2=CH—CH2CH3
g. CH3COCH3
h. CH3CH2COOH
Problem 32a,b
Predict which member of each pair is more soluble in water. Explain your prediction.
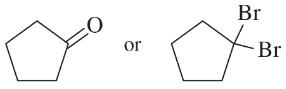
(a)
(b)
Problem 32c,d
Predict which member of each pair is more soluble in water. Explain your prediction.
(c)
(d)
Problem 32e,f
Predict which member of each pair is more soluble in water. Explain your prediction.
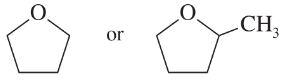
(e)
(f)
Problem 33
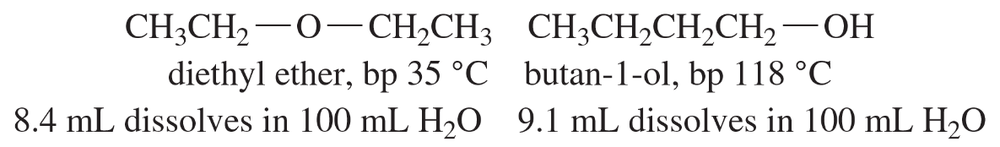
Diethyl ether and butan-1-ol are isomers, and they have similar solubilities in water. Their boiling points are very different, however. Explain why these two compounds have similar solubility properties but dramatically different boiling points.
Problem 34b
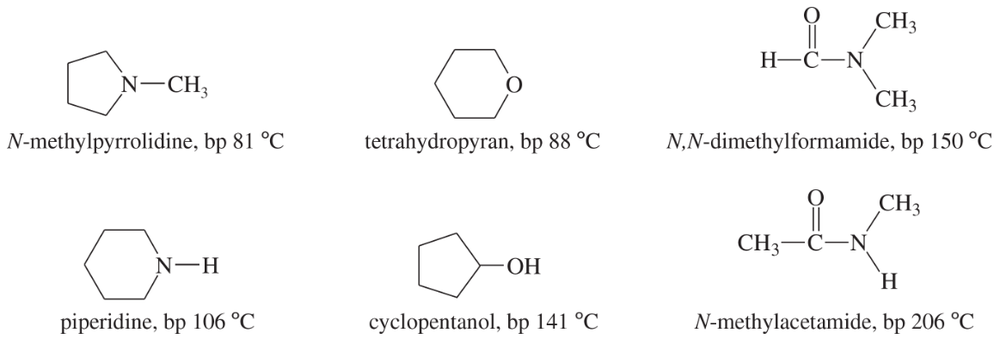
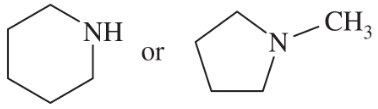
N-Methylpyrrolidine has a boiling point of 81 °C, and piperidine has a boiling point of 106 °C.
b. Tetrahydropyran has a boiling point of 88 °C, and cyclopentanol has a boiling point of 141 °C. These two isomers have a boiling point difference of 53 °C. Explain why the two oxygen-containing isomers have a much larger boiling point difference than the two amine isomers.
Problem 34c
N-Methylpyrrolidine has a boiling point of 81 °C, and piperidine has a boiling point of 106 °C.
c. N,N-Dimethylformamide has a boiling point of 150 °C, and N-methylacetamide has a boiling point of 206 °C, for a difference of 56 °C. Explain why these two nitrogen-containing isomers have a much larger boiling point difference than the two amine isomers. Also explain why these two amides have higher boiling points than any of the other four compounds shown (two amines, an ether, and an alcohol).
Problem 35a,b
Predict which compound in each pair has the higher boiling point. Explain your prediction.
(a) CH3CH2OCH3 or CH3CH(OH)CH3
(b) CH3CH2CH2CH3 or CH3CH2CH2CH2CH3
Problem 35c,d
Predict which compound in each pair has the higher boiling point. Explain your prediction.
(c) CH3CH2CH2CH2CH3 or (CH3)2CH2CH2CH3
(d) CH3CH2CH2CH2CH3 or CH3CH2CH2CH2CH2Cl
Problem 35e,f
Predict which compound in each pair has the higher boiling point. Explain your prediction.
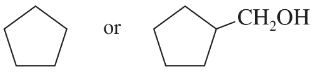
(e)
(f)
Problem 36
Rank the following species in order of increasing acidity. Explain your reasons for ordering them as you do.
HF NH3 H2SO4 CH3OH CH3COOH H3O+ H2O
Problem 36a
All of the following compounds can react as acids. Without using a table of acidities, rank them in order of increasing acidity. Explain your ranking.
a. CH3CH2SO3H
b. CH3CH2OH
c. CH3CH2COOH
d. CH3CHClCOOH
e. ClCH2CH2COOH
Problem 38
Rank the following species in order of increasing basicity. Explain your reasons for ordering them as you do.
NH3 CH3O– H2O CH3COO– NaOH NH2– HSO4–
Problem 39a,b
The Ka of phenylacetic acid is 5.2 × 10−5, and the pKa of propionic acid is 4.87.
a. Calculate the pKa of phenylacetic acid and the Ka of propionic acid.
b. Which of these is the stronger acid? Calculate how much stronger an acid it is.
Problem 39c
The Ka of phenylacetic acid is 5.2 × 10−5, and the pKa of propionic acid is 4.87.
c. Predict whether the following equilibrium will favor the reactants or the products.
Problem 40a
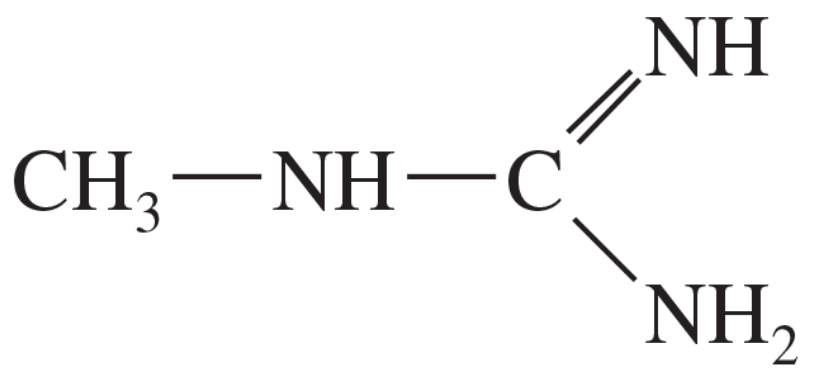
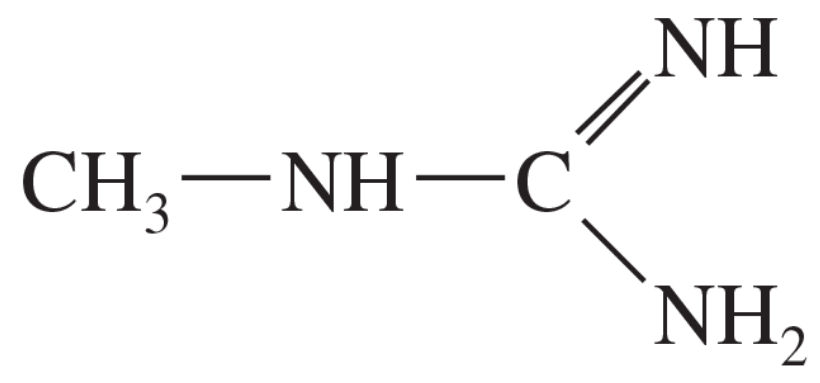
The following compound can become protonated on any of the three nitrogen atoms. One of these nitrogens is much more basic than the others, however.
a. Draw the important resonance forms of the products of protonation on each of the three nitrogen atoms.
Problem 40b
The following compound can become protonated on any of the three nitrogen atoms. One of these nitrogens is much more basic than the others, however.
b. Determine which nitrogen atom is the most basic.
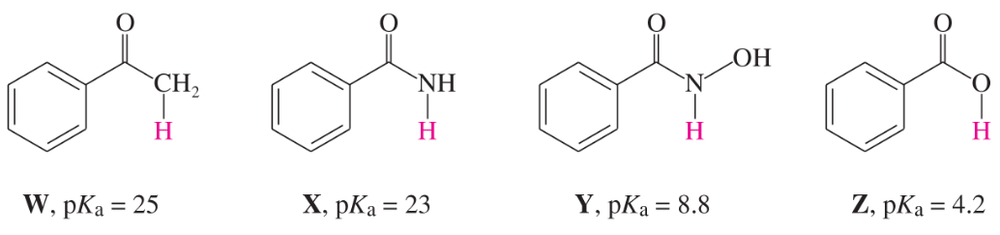
Problem 41b,c,d
The following compounds are listed in increasing order of acidity. In each case, the most acidic proton is shown in red.
b. Explain why X is a stronger acid than W.
c. Explain why Y is a stronger acid than X.
d. Explain why Z is a stronger acid than Y.
Problem 42a,b
Predict the products of the following acid–base reactions.
(a) H2SO4 + CH3COO– ⇌
(b) CH3COOH + (CH3)3N: ⇌
Problem 42a
Hydrogen peroxide (HOOH) has a pKa of 11.6, making it roughly 10,000 times as strong an acid as water (pKa = 15.7). Explain why H2O2 is a stronger acid than H2O.
Problem 42b
Peroxyacetic acid (pKa = 8.2) is a much weaker acid than acetic acid (pKa = 4.74). Explain why peroxyacetic acid is a weaker acid than acetic acid.
Problem 42c,d
Predict the products of the following acid–base reactions.
(c)
(d)
Problem 42e
Predict the products of the following acid–base reactions.
(e) H2O + NH3 ⇌
Problem 42f
Predict the products of the following acid–base reactions.
(f)
Problem 42g,h
Predict the products of the following acid–base reactions.
(g) HCOOH + CH3O– ⇌
(h) +NH3CH2COOH + 2 –OH ⇌
Problem 42g
Predict the products of the following acid–base reactions.
(g) HCOOH + CH3O– ⇌
Problem 42h
Predict the products of the following acid–base reactions.
(h)
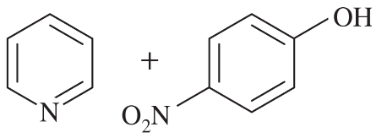
Problem 43a,b
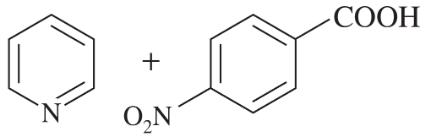
Consider the following proposed Brønsted–Lowry acid–base reactions. In each case, draw the products of a transfer of the most acidic proton on the acid to the most basic site on the base. Use Appendix 4 to find or estimate the pKa values for the acids and the pKb values for the bases. Then determine which side of the reaction is favored, either reactants or products.
(a)
(b)
Problem 44
Compare the relative acidity of 1-molar aqueous solutions of the following acids.
Problem 45a
The following compounds can all react as acids.
a. For each compound, show its conjugate base. Show any resonance forms if applicable. b. Rank the conjugate bases in the order you would predict, from most stable to least stable.