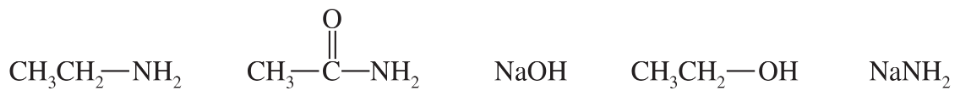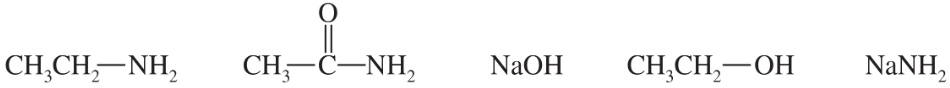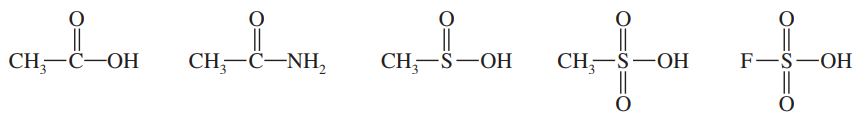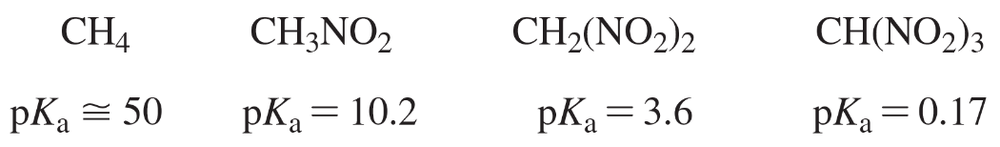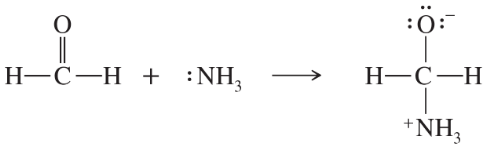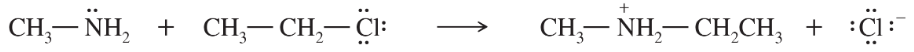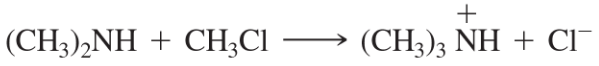Back
BackProblem 45c
The following compounds can all react as acids.
c. Rank the original compounds in order, from strongest acid to weakest acid.
Problem 46a
The following compounds can all react as bases.
a. For each compound, show its conjugate acid. Show any resonance forms if applicable.
Problem 46b
The following compounds can all react as bases.
b. Rank the conjugate acids in the order you would predict, from most stable to least stable.
Problem 46c
The following compounds can all react as bases.
c. Rank the original compounds in order, from strongest base to weakest base.
Problem 47a
The following compounds can all react as acids.
a. For each compound, show its conjugate base. Show any resonance forms if applicable.
Problem 47b
The following compounds can all react as acids.
b. Rank the conjugate bases in the order you would predict, from most stable to least stable.
Problem 48
Consider the following compounds that vary from nearly nonacidic to strongly acidic. Draw the conjugate bases of these compounds, and explain why the acidity increases so dramatically with substitution by nitro groups.
Problem 49b
Methyllithium (CH3Li) is often used as a base in organic reactions.
b. What is the conjugate acid of CH3Li? Would you expect CH3Li to be a strong base or a weak base?
Problem 50a
Label the reactants in these acid–base reactions as Lewis acids (electrophiles) or Lewis bases (nucleophiles). Use curved arrows to show the movement of electron pairs in the reactions.
(a)
Problem 50b
Label the reactants in these acid–base reactions as Lewis acids (electrophiles) or Lewis bases (nucleophiles). Use curved arrows to show the movement of electron pairs in the reactions.
(b)
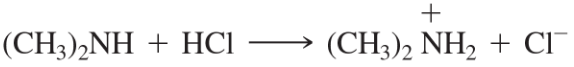
Problem 50c
Label the reactants in these acid–base reactions as Lewis acids (electrophiles) or Lewis bases (nucleophiles). Use curved arrows to show the movement of electron pairs in the reactions.
(c)
Problem 50d
Label the reactants in these acid–base reactions as Lewis acids (electrophiles) or Lewis bases (nucleophiles). Use curved arrows to show the movement of electron pairs in the reactions.
(d)
Problem 50i
Label the reactants in these acid–base reactions as Lewis acids (electrophiles) or Lewis bases (nucleophiles). Use curved arrows to show the movement of electron pairs in the reactions.
i.
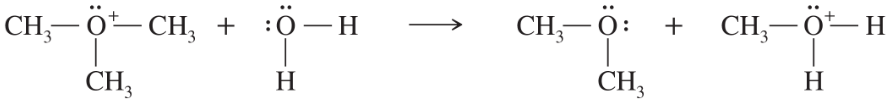
Problem 51a
In each reaction, label the reactants as Lewis acids (electrophiles) or Lewis bases (nucleophiles). Use curved arrows to show the movement of electron pairs in the reactions. Draw any nonbonding electrons to show how they participate in the reactions.
(a)
Problem 51b
In each reaction, label the reactants as Lewis acids (electrophiles) or Lewis bases (nucleophiles). Use curved arrows to show the movement of electron pairs in the reactions. Draw any nonbonding electrons to show how they participate in the reactions.
(b)
Problem 51c
In each reaction, label the reactants as Lewis acids (electrophiles) or Lewis bases (nucleophiles). Use curved arrows to show the movement of electron pairs in the reactions. Draw any nonbonding electrons to show how they participate in the reactions.
c.
Problem 52a,b
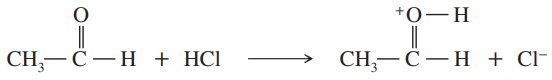
Each of these compounds can react as an electrophile. In each case, use curved arrows to show how the electrophile would react with the strong nucleophile sodium ethoxide, Na+ −OCH2CH3.
a.
b. NH4+
Problem 52c,d
Each of these compounds can react as an electrophile. In each case, use curved arrows to show how the electrophile would react with the strong nucleophile sodium ethoxide, Na+ −OCH2CH3.
(c) CH3CH2Br
(d) BH3
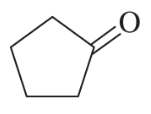
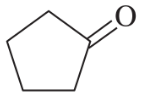
Problem 53a,b
Each of these compounds can react as a nucleophile. In each case, use curved arrows to show how the nucleophile would react with the strong electrophile BF3.
(a)
(b)
Problem 53e
Each of these compounds can react as a nucleophile. In each case, use curved arrows to show how the nucleophile would react with the strong electrophile BF3.
(e) CH3CH2OH
Problem 53f
Each of these compounds can react as a nucleophile. In each case, use curved arrows to show how the nucleophile would react with the strong electrophile BF3.
(f) (CH3)2S
Problem 54a,b
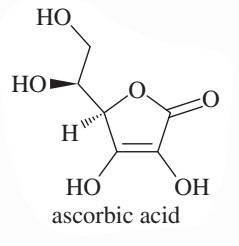
The pKa of ascorbic acid (vitamin C, page 55) is 4.17, showing that it is slightly more acidic than acetic acid (CH3COOH, pKa 4.74).
a. Show the four different conjugate bases that would be formed by deprotonation of the four different OH groups in ascorbic acid.
b. Compare the stabilities of these four conjugate bases, and predict which OH group of ascorbic acid is the most acidic.
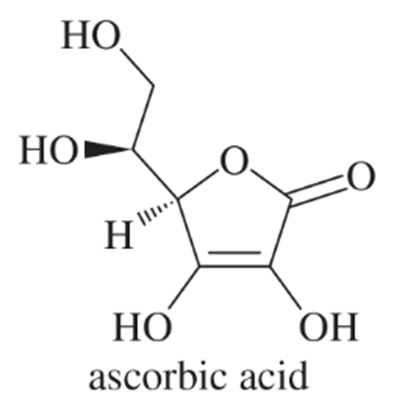
Problem 54c
The pKa of ascorbic acid (vitamin C, page 55) is 4.17, showing that it is slightly more acidic than acetic acid (CH3COOH, pKa 4.74).
c. Compare the most stable conjugate base of ascorbic acid with the conjugate base of acetic acid, and suggest why these two compounds have similar acidities, even though ascorbic acid lacks the carboxylic acid (COOH) group.
Problem 57a,b
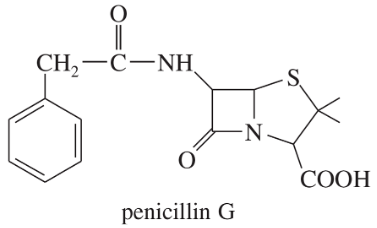
Many naturally occurring compounds contain more than one functional group. Identify the functional groups in the following compounds:
a. Penicillin G is a naturally occurring antibiotic.
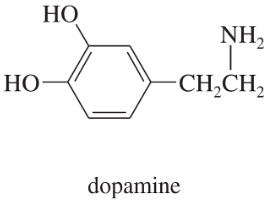
b. Dopamine is the neurotransmitter that is deficient in Parkinson’s disease.
Problem 57c
Many naturally occurring compounds contain more than one functional group. Identify the functional groups in the following compounds:
d. Thyroxine is the principal thyroid hormone.