Back
BackProblem 36
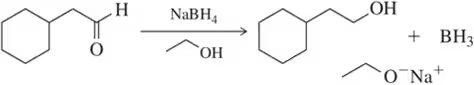
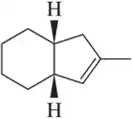
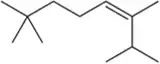
Because deuterium behaves like hydrogen in chemical reactions yet is detected differently, chemists use the incorporation of deuterium to better understand the subtleties of reaction mechanisms. Deuterium is incorporated by replacing H₂ with D₂ in the hydrogenation reaction. Identify the product expected when the alkenes in Assessment 9.34 react with D₂ and Pd/C. [Don't worry about showing all diastereomers.]
Problem 37a
Without concerning yourself with the mechanism of the reaction, calculate the equilibrium constant for the following equilibrium processes. (Assume T = 298 K.)
(a)
Problem 38a
Conjugated dienes, molecules containing two alkenes separated by one single bond, are discussed in detail in Chapter 21.
(a) Considering the observed ∆H° of hydrogenation, is hexa-1,3-diene (conjugated) or hexa-1,4-diene (unconjugated) more stable?
Problem 38b
Conjugated dienes, molecules containing two alkenes separated by one single bond, are discussed in detail in Chapter 21.
(b) How might you account for this difference in stability?
Problem 39c
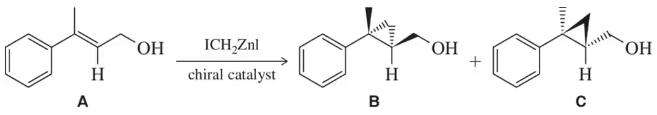
Questions (a)–(d) all refer to the following reaction, which has been engineered to produce one enantiomer to the exclusion of the other.
(c) Suppose the difference in activation energy is 1.6 kcal/mol. At what temperature would you produce C in 99% ee?
Problem 40a
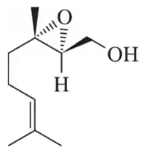
Identify the alkene that would react with Ti(OiPr)₄, (+) -diethyltartrate, and t-butylhydroperoxide to give the following chiral, nonracemic epoxides.
(a)
Problem 40b
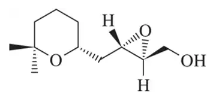
Identify the alkene that would react with Ti(OiPr)4, (+) -diethyltartrate, and t-butylhydroperoxide to give the following chiral, nonracemic epoxides.
(b)
Problem 40c
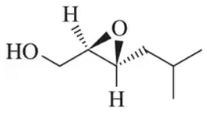
Identify the alkene that would react with Ti(OiPr)₄, (+) -diethyltartrate, and t-butylhydroperoxide to give the following chiral, nonracemic epoxides.
(c)
Problem 41
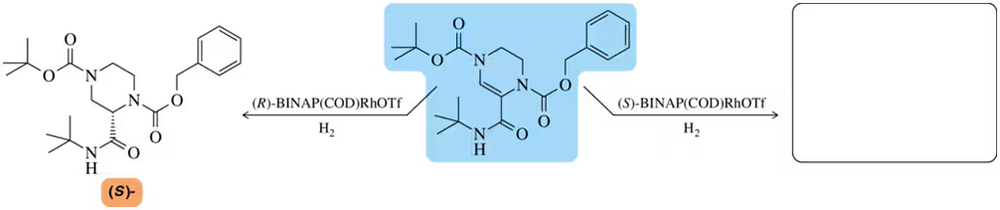
The chiral catalyst (R)-BINAP(COD)RhOTf was used in a hydrogenation reaction as part of the synthesis of fragment C of indinavir. Using the same alkene, predict the product that would be obtained if (S)-BINAP(COD)RhOTf were used instead.
Problem 43a
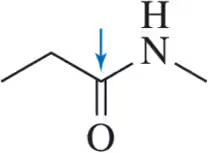
Calculate the oxidation number for the indicated carbons.
(a)
Problem 43b
Calculate the oxidation number for the indicated carbons.
(b)
Problem 43c
Calculate the oxidation number for the indicated carbons.
(c)
Problem 43d
Calculate the oxidation number for the indicated carbons.
(d)
Problem 44a
Determine whether the following reactions are redox reactions. If they are, identify whether the molecule has been oxidized or reduced.
(a)
Problem 44b
Determine whether the following reactions are redox reactions. If they are, identify whether the molecule has been oxidized or reduced.
(b)
Problem 44c
Determine whether the following reactions are redox reactions. If they are, identify whether the molecule has been oxidized or reduced.
(c)
Problem 44d
Determine whether the following reactions are redox reactions. If they are, identify whether the molecule has been oxidized or reduced.
(d)
Problem 45d(i)
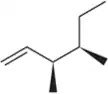
Predict the product(s) that would result when the alkenes are allowed to react under the following conditions: (i) Br2
(d)
Problem 45d(ii)
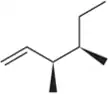
Predict the product(s) that would result when the alkenes are allowed to react under the following conditions: (ii) Cl2
(d)
Problem 45d(iii)
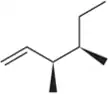
Predict the product(s) that would result when the alkenes are allowed to react under the following conditions: (iii) Br2, H2O
(d)
Problem 45d(iv)
Predict the product(s) that would result when the alkenes are allowed to react under the following conditions: (iv) Cl2, CH3OH
(d)
Problem 45d(v)
Predict the product(s) that would result when the alkenes are allowed to react under the following conditions: (v) mCPBA
(d)
Problem 45d(vi)
Predict the product(s) that would result when the alkenes are allowed to react under the following conditions: (vi) 1. OsO4 2. NaHSO3
(d)
Problem 45d(vii)
Predict the product(s) that would result when the alkenes are allowed to react under the following conditions: (vii) 1. mCPBA 2. H2SO4, H2O
(d)
Problem 45d(viii)
Predict the product(s) that would result when the alkenes are allowed to react under the following conditions: (viii) 1. O3 2. CH3SCH3
(d)
Problem 45d(ix)
Predict the product(s) that would result when the alkenes are allowed to react under the following conditions: (ix) H2, Pd/C;
(d)
Problem 45d(x)
Predict the product(s) that would result when the alkenes are allowed to react under the following conditions: (x) D2, Pd/C.
(d)
Problem 45e(v)
Predict the product(s) that would result when the alkenes are allowed to react under the following conditions: (v) mCPBA;
(k)
Problem 45e(i,ii)
Predict the product(s) that would result when the alkenes are allowed to react under the following conditions: (i) Br2 (ii) Cl2.
(e)
Problem 45e(iii,iv)
Predict the product(s) that would result when the alkenes are allowed to react under the following conditions: (iii) Br2, H2O ; (iv) Cl2, CH3OH.
(e)