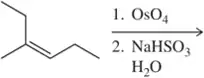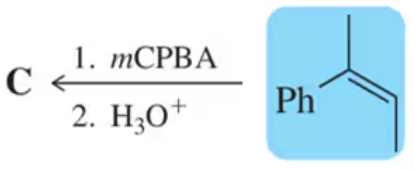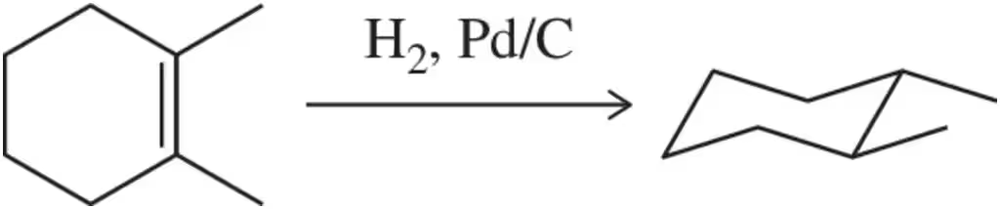Back
BackProblem 16a
Predict the product(s) when each of the following are reacted with mCPBA, making sure to indicate the relative stereochemical outcome. Indicate any racemic mixtures by drawing both enantiomers.
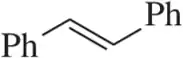
(a)
Problem 16d
Predict the product(s) when each of the following are reacted with mCPBA, making sure to indicate the relative stereochemical outcome. Indicate any racemic mixtures by drawing both enantiomers.
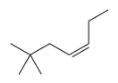
(d)
Problem 17
Evidence for the concertedness of epoxide formation comes from the stereospecificity of the reaction. If step 2 of a hypothetical stepwise mechanism were slow enough to make the reaction non-concerted, how would the product distribution change?
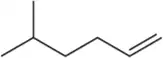
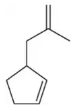
Problem 17.8a
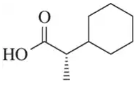
Predict the product of the following aldehyde/ketone syntheses.
(a) <IMAGE>
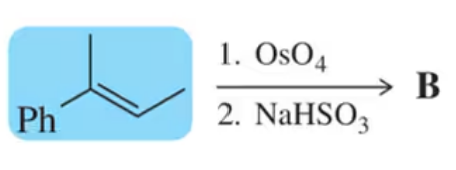
Problem 18b
When producing a chiral molecule, epoxide formation still results in a mixture of enantiomers, despite its stereospecificity.
(b) How is it that a reaction can be stereospecific while still producing two enantiomers?
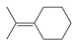
Problem 19
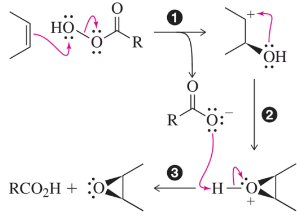
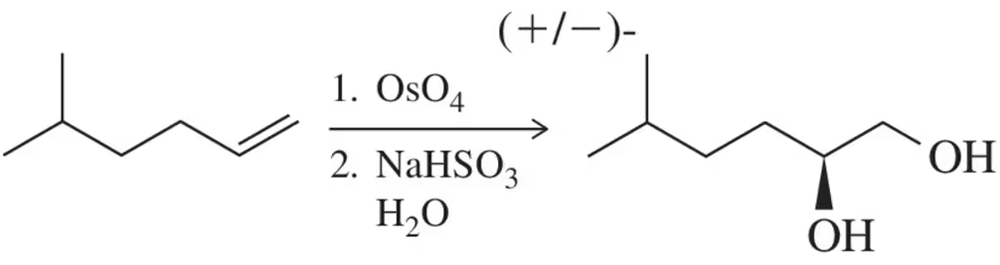
How might you make the catalytic cycle in Figure 9.31 more sustainable while still using NMO as the co-oxidant?
<IMAGE>
Problem 20b
Predict the product(s) of each of the following reactions, making sure to indicate the relative stereochemical outcome. Indicate any racemic mixtures by drawing both enantiomers.
(b)
Problem 20c
Predict the product(s) of each of the following reactions, making sure to indicate the relative stereochemical outcome. Indicate any racemic mixtures by drawing both enantiomers.
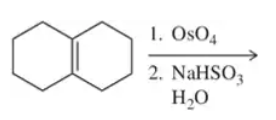
(c)
Problem 21
When using a terminal alkene under the conditions shown here, explain why it is unnecessary to show the relative stereochemical outcome in the product.
Problem 22c
Which of molecules A–D would you expect to give a positive permanganate test? That is, which would result in a purple KMnO₄ solution turning brown?
(c)
Problem 22d
Which of molecules A–D would you expect to give a positive permanganate test? That is, which would result in a purple KMnO₄ solution turning brown?
(d)
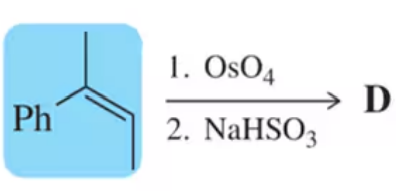
Problem 23
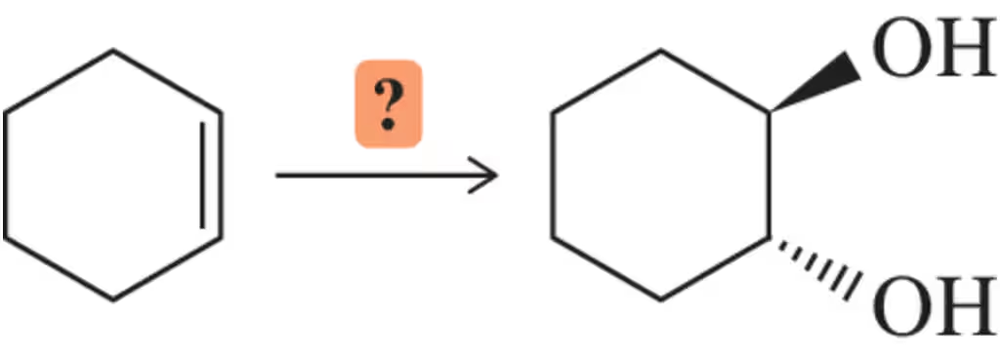
The concertedness of the OsO4 reaction results in both oxygens being added to the same face of the molecule (i.e., syn addition). How might these conditions be modified in order to prepare a trans-diol?
Problem 24
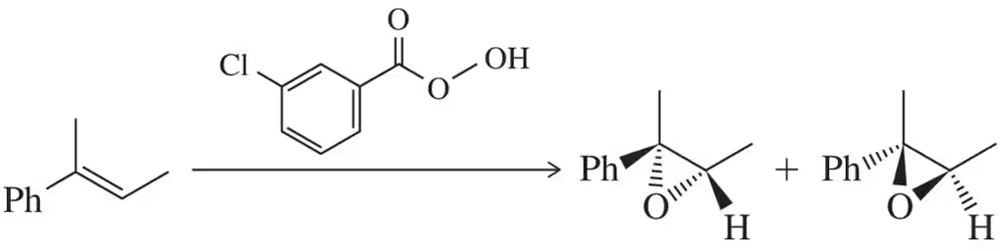
There are two mechanisms by which each of the two enantiomers can form in the reaction shown in Figure 9.37. Show them.
<IMAGE>
Problem 25
Show an arrow-pushing mechanism for reactions 1–4 in Figure 9.37.
<IMAGE>
Problem 26a
Predict the product(s) of each of the following reactions, making sure to indicate the relative stereochemical outcome. Indicate any racemic mixtures by drawing both enantiomers.
(a)
Problem 26b
Predict the product(s) of each of the following reactions, making sure to indicate the relative stereochemical outcome. Indicate any racemic mixtures by drawing both enantiomers.
(b)
Problem 26c
Predict the product(s) of each of the following reactions, making sure to indicate the relative stereochemical outcome. Indicate any racemic mixtures by drawing both enantiomers.
(c)
Problem 26d
Predict the product(s) of each of the following reactions, making sure to indicate the relative stereochemical outcome. Indicate any racemic mixtures by drawing both enantiomers.
(d)
Problem 27
In Assessment 9.26, what is the relationship between the enantiomers of compound A and the enantiomers of compound B?
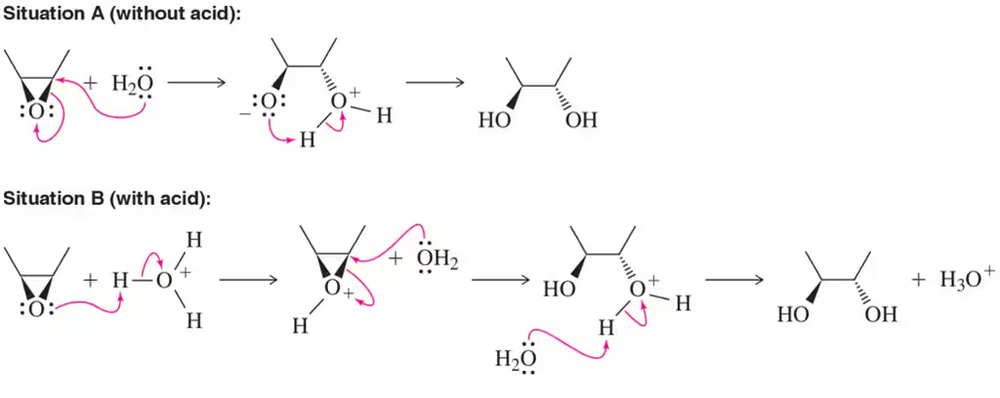
Problem 28
Despite it being equally favorable, opening of the epoxide does not happen in the absence of an acid catalyst. How does acid make the reaction faster? Demonstrate this concept by directly comparing the reaction coordinate diagram for both situations A and B.
Problem 29a
Draw the product(s) you'd expect when each of these alkenes is treated first with O3, then with CH3SCH3
(a)
Problem 29b
Draw the product(s) you'd expect when each of these alkenes is treated first with O3, then with CH3SCH3
(b)
Problem 29c
Draw the product(s) you'd expect when each of these alkenes is treated first with O3, then with CH3SCH3
(c)
Problem 29d
Draw the product(s) you'd expect when each of these alkenes is treated first with O3, then with CH3SCH3
(d)
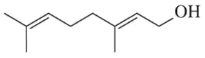
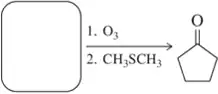
Problem 30a
Suggest a synthesis of the following aldehydes or ketones using the ozonolysis reaction of an alkene.
(a)
Problem 30b
Suggest a synthesis of the following aldehydes or ketones using the ozonolysis reaction of an alkene.
(b)
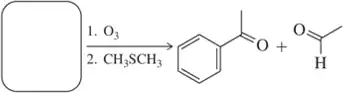
Problem 31
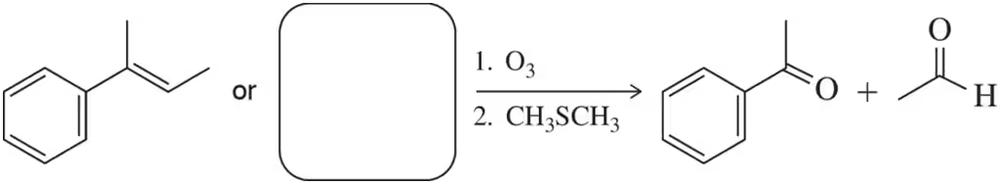
In Solved Assessment 9.30(b), we came up with an alkene that under the conditions of ozonolysis would produce acetophenone and acetaldehyde. There is one other alkene that should produce the same compounds under these conditions. Which is it?
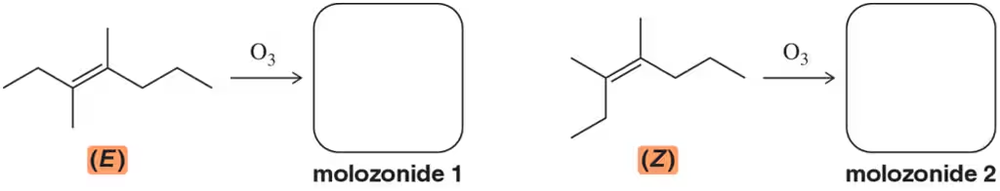
Problem 33
Formation of the molozonide can be expected to proceed stereospecifically. Why is this the case? Show the two different molozonides you would expect to get from ozonolysis of (E)- and (Z)-3,4-dimethylhept-3-ene.
Problem 34b
What product results when the following molecules are treated with H₂ Pd/C? Be sure to indicate the relative stereochemical outcome. Draw both enantiomers of any racemic mixtures. [It is difficult to control the stoichiometry of gases so there is enough H₂ to reduce all alkenes.]
(b)
Problem 35
A misguided chemist attempted to synthesize trans-1,2-dimethylcyclohexane via the hydrogenation of 1,2-dimethylcyclohexene. Explain why this is not possible.