Back
BackProblem 45l(vii,viii)
Predict the product(s) that would result when the alkenes are allowed to react under the following conditions: (vii) 1. mCPBA 2. H2SO4, H2O (viii) 1. O3 2. CH3SCH3
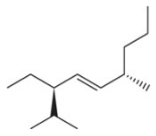
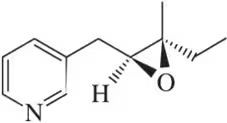
(l)
Problem 46
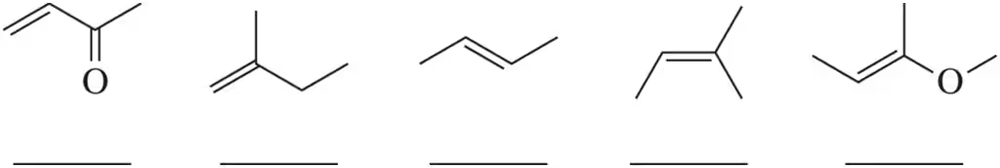
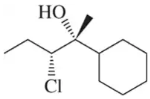
Rank the reactivity of the following alkenes with mCPBA ( 1 = most reactive , 5 least reactive ).
Problem 47
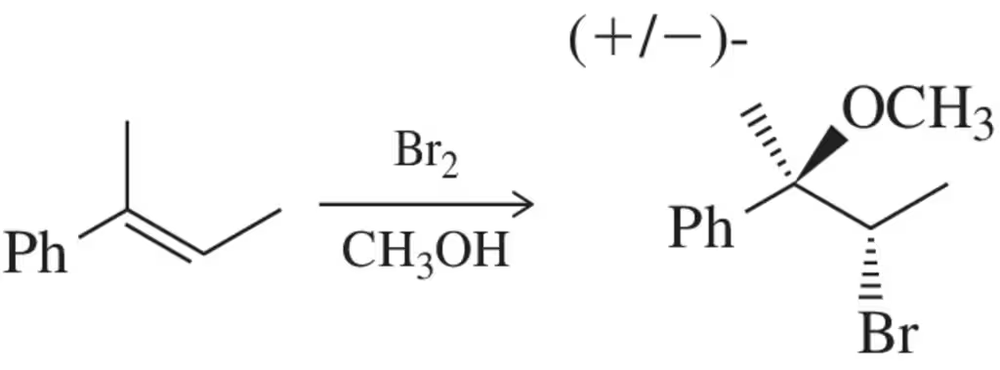
When alkenes react with bromine in water, a halohydrin is produced. When water is replaced with methanol in this reaction, a different product is produced. Suggest a mechanism for the formation of this product.
Problem 48a
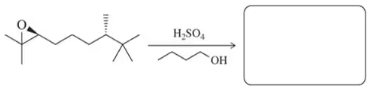
In light of your answer to Assessment 9.47, predict the product of the following reactions we have seen previously where an alcohol is substituted for water.
(a)
Problem 48b
In light of your answer to Assessment 9.47, predict the product of the following reactions we have seen previously where an alcohol is substituted for water.
(b)
Problem 48c
In light of your answer to Assessment 9.47, predict the product of the following reactions we have seen previously where an alcohol is substituted for water.
(c)
Problem 50a
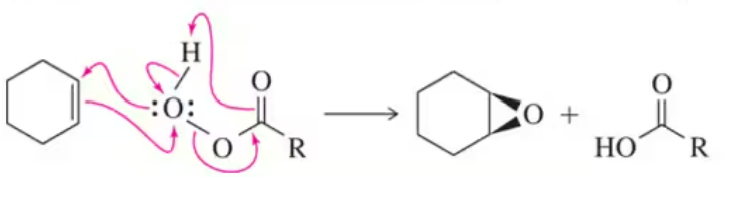
One way to think about concerted reactions is to imagine them as being stepwise reactions where, besides the slowest step, all others have infinitesimally small activation energies. Considering the hypothetical stepwise mechanism and actual concerted mechanism of epoxide formation, show what a reaction coordinate diagram might look like for each possibility.
(a) Stepwise, hypothetical mechanism:
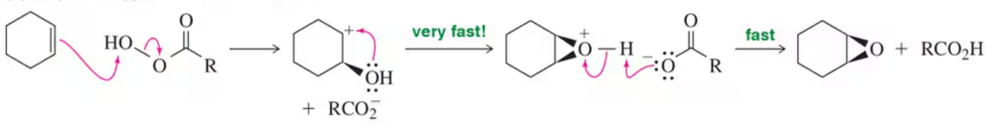
Problem 50b
One way to think about concerted reactions is to imagine them as being stepwise reactions where, besides the slowest step, all others have infinitesimally small activation energies. Considering the hypothetical stepwise mechanism and actual concerted mechanism of epoxide formation, show what a reaction coordinate diagram might look like for each possibility.
(b) Concerted , actual mechanism (butterfly transition state:
Problem 51
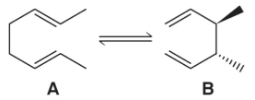
Consider the Cope rearrangement, a reaction we describe in Chapter 20.
(a) Using the knowledge we have gained here in Chapter 9, suggest a one-step, concerted mechanism that explains the formation of B from A.
(b) Which side of the reaction would you expect to be favored? Justify your answer.
(c) Which product, A or B, would you expect to be hydrogenated with the more exothermic heat of hydrogenation?
Problem 52a
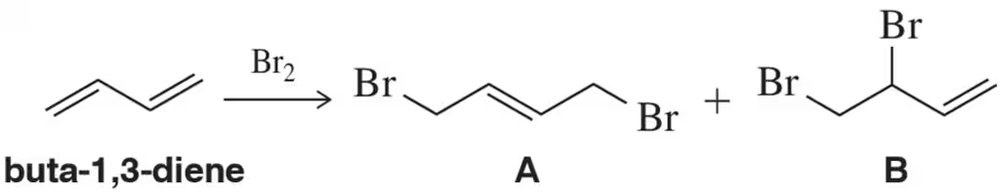
Bromination of buta-1,3-diene with a single equivalent of Br2 can give either of two products. (a) Which of these products (A or B) would you predict to be more stable? Justify your answer.
Problem 53
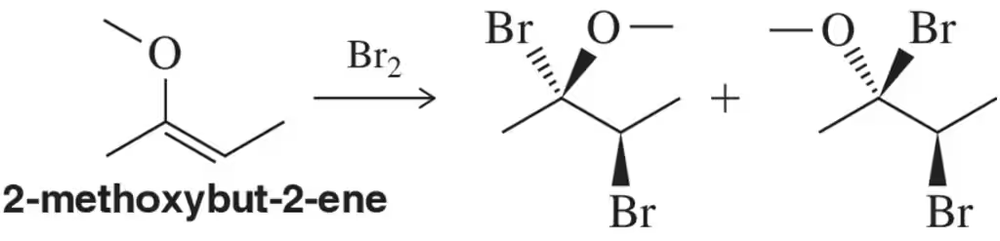
Bromination of a highly electron-rich alkene such as 2-methoxybut-2-ene has been shown to produce approximately equal mixtures of the trans- and cis-dibromide. Suggest an explanation for this observation.
Problem 54
In Chapter 19, we discuss the reaction of enols with bromine. This reaction produces α -bromoketones in good yields. Suggest a mechanism for this reaction and justify its deviation from the dibromide product you might have expected.
Problem 55
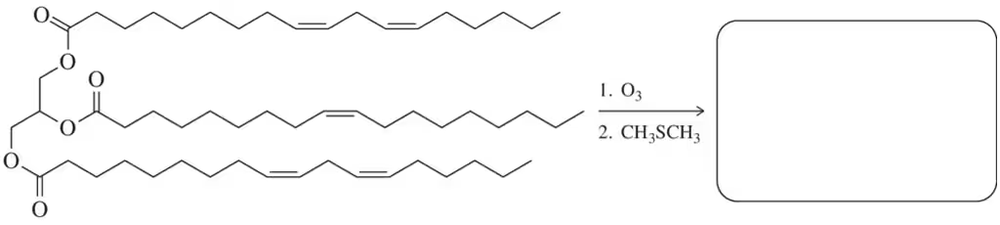
Predict the product of ozonolysis of the triglyceride shown.
Problem 56
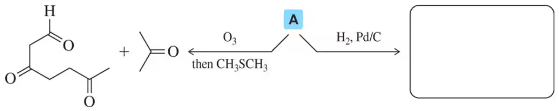
Ozonolysis of an unknown alkene A gives the products shown. Predict the product that results from hydrogenation of alkene A. [There are multiple answers, but only show the one with the 6-membered ring.]
Problem 57
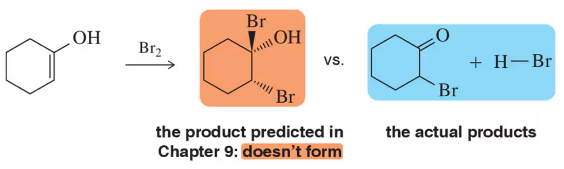
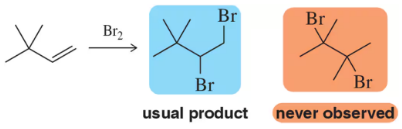
In spite of being mechanistically similar to some of the reactions we saw in Chapter 8, rearrangement never occurred here in Chapter 9. Why doesn't rearrangement occur in the following bromination reaction despite the proximity of a more substituted carbon?
Problem 59a
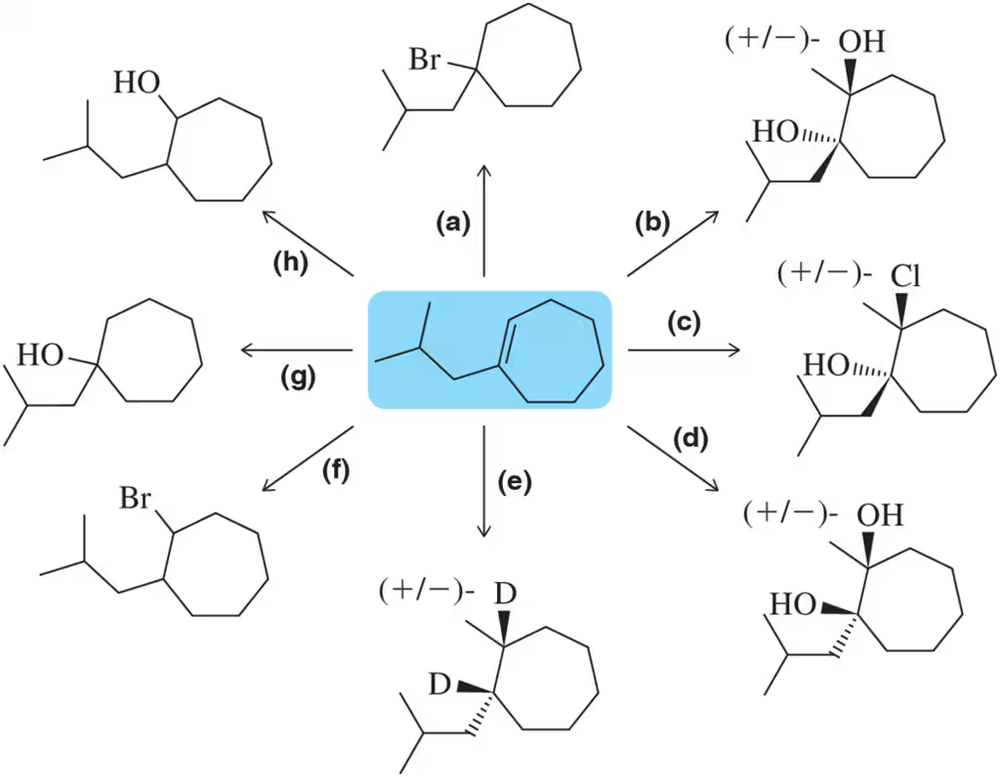
At the beginning of Chapter 9, we stated that after finishing Chapters 8 and 9, we would have the ability to make a large variety of functional groups using related reactions. Show the reagent(s) necessary to convert 1-isobutylcyclohexene into the following molecules.
(a)
Problem 59b
At the beginning of Chapter 9, we stated that after finishing Chapters 8 and 9, we would have the ability to make a large variety of functional groups using related reactions. Show the reagent(s) necessary to convert 1-isobutylcyclohexene into the following molecules.
(b)
Problem 59c
At the beginning of Chapter 9, we stated that after finishing Chapters 8 and 9, we would have the ability to make a large variety of functional groups using related reactions. Show the reagent(s) necessary to convert 1-isobutylcyclohexene into the following molecules.
(c)
Problem 59d
At the beginning of Chapter 9, we stated that after finishing Chapters 8 and 9, we would have the ability to make a large variety of functional groups using related reactions. Show the reagent(s) necessary to convert 1-isobutylcyclohexene into the following molecules.
(d)
Problem 59e
At the beginning of Chapter 9, we stated that after finishing Chapters 8 and 9, we would have the ability to make a large variety of functional groups using related reactions. Show the reagent(s) necessary to convert 1-isobutylcyclohexene into the following molecules.
(e)
Problem 59f
At the beginning of Chapter 9, we stated that after finishing Chapters 8 and 9, we would have the ability to make a large variety of functional groups using related reactions. Show the reagent(s) necessary to convert 1-isobutylcyclohexene into the following molecules.
(f)
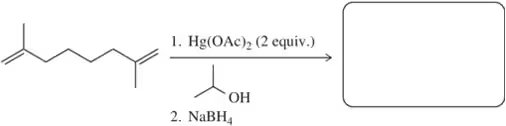
Problem 59g
At the beginning of Chapter 9, we stated that after finishing Chapters 8 and 9, we would have the ability to make a large variety of functional groups using related reactions. Show the reagent(s) necessary to convert 1-isobutylcyclohexene into the following molecules.
(g)
Problem 59h
At the beginning of Chapter 9, we stated that after finishing Chapters 8 and 9, we would have the ability to make a large variety of functional groups using related reactions. Show the reagent(s) necessary to convert 1-isobutylcyclohexene into the following molecules.
(h)
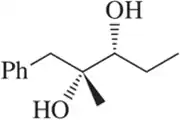
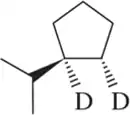
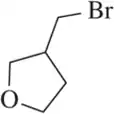
Problem 60a
Retrosynthetic analysis is the process of working backward to develop the synthesis of a new compound. In Chapter 10, we begin developing multistep syntheses in this manner. For now, try to work backward a single step by suggesting an alkene and a reagent that would give products (a)–(i). [Your answers should not include alkenes that undergo rearrangement to give the desired products.]
(a)
Problem 60b
Retrosynthetic analysis is the process of working backward to develop the synthesis of a new compound. In Chapter 10, we begin developing multistep syntheses in this manner. For now, try to work backward a single step by suggesting an alkene and a reagent that would give products (a)–(i). [Your answers should not include alkenes that undergo rearrangement to give the desired products.]
(b)
Problem 60d
Retrosynthetic analysis is the process of working backward to develop the synthesis of a new compound. In Chapter 10, we begin developing multistep syntheses in this manner. For now, try to work backward a single step by suggesting an alkene and a reagent that would give products (a)–(i). [Your answers should not include alkenes that undergo rearrangement to give the desired products.]
(d)
Problem 60f
Retrosynthetic analysis is the process of working backward to develop the synthesis of a new compound. In Chapter 10, we begin developing multistep syntheses in this manner. For now, try to work backward a single step by suggesting an alkene and a reagent that would give products (a)–(i). [Your answers should not include alkenes that undergo rearrangement to give the desired products.]
(f)
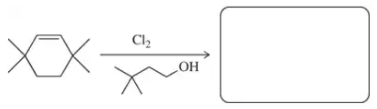
Problem 60i
Retrosynthetic analysis is the process of working backward to develop the synthesis of a new compound. In Chapter 10, we begin developing multistep syntheses in this manner. For now, try to work backward a single step by suggesting an alkene and a reagent that would give products (a)–(i). [Your answers should not include alkenes that undergo rearrangement to give the desired products.]
(i)
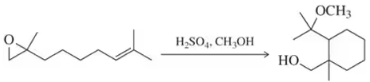
Problem 61a
Suggest mechanisms for the following reactions, which are similar to the mechanism we saw for lanosterol biosynthesis at the end of Chapter 8.
(a)
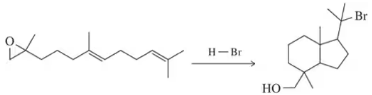
Problem 61b
Suggest mechanisms for the following reactions, which are similar to the mechanism we saw for lanosterol biosynthesis at the end of Chapter 8.
(b)