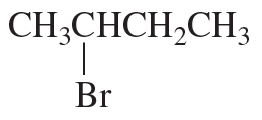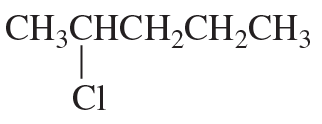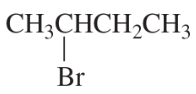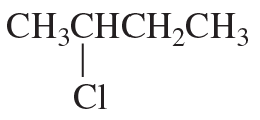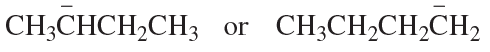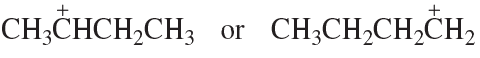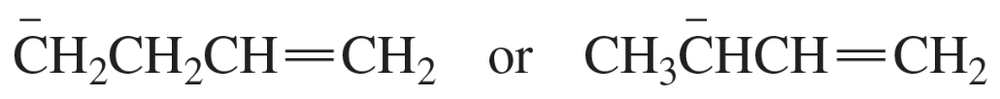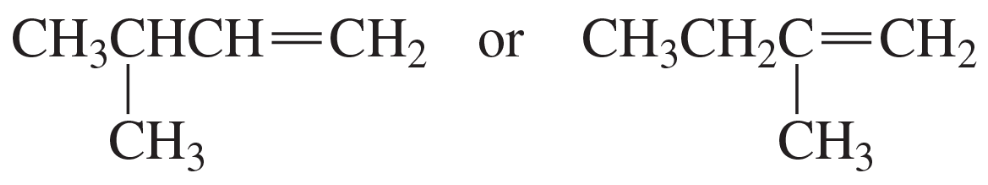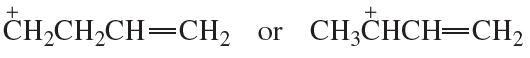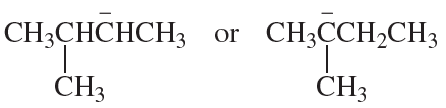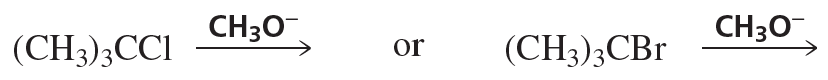Back
BackProblem 77c,d
Draw the substitution products for each of the following reactions; if the products can exist as stereoisomers, show what stereoisomers are obtained:
c. benzyl chloride + CH3CH2OH
d. allyl chloride + CH3OH
Problem 77e,f
Draw the substitution products for each of the following reactions; if the products can exist as stereoisomers, show what stereoisomers are obtained:
e. 1-bromo-2-butene + CH3O−
f. 1-bromo-2-butene + CH3OH
Problem 79
Would you expect methoxide ion to be a better nucleophile if it is dissolved in CH3OH or if it is dissolved in DMSO? Why?
Problem 81
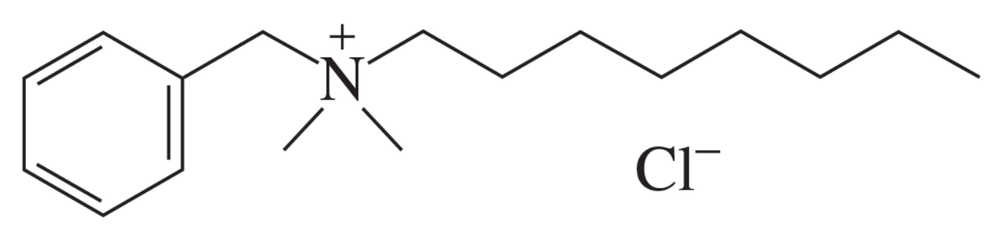
Alkylbenzyldimethyl ammonium chloride is a leave-on skin antiseptic used to treat such things as cuts and cold sores. It is also the antiseptic in many hand sanitizers. It is actually a mixture of compounds that differ in the number of carbons (any even number between 8 and 18) in the alkyl group. Show three different sets of reagents (each set composed of an alkyl chloride and an amine) that can be used to synthesize the alkylbenzyldimethyl ammonium chloride shown here.
Problem 82a,b
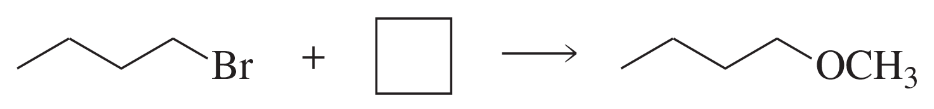
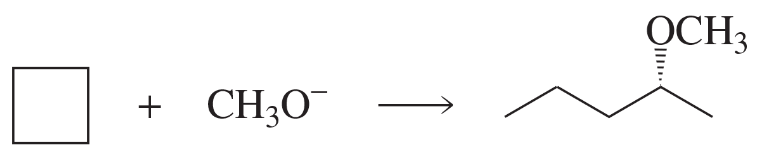
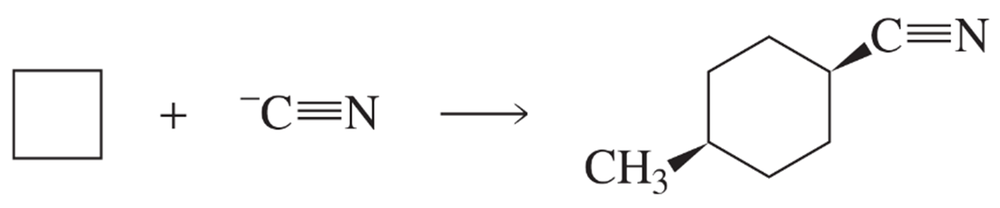
Fill in the squares in the following chemical equations:
a.
b.
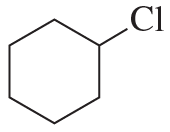
Problem 82c,d
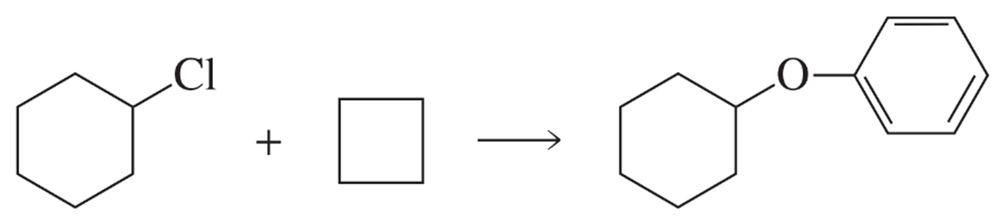
Fill in the squares in the following chemical equations:
c.
d.
Problem 83a
Draw the major product obtained when each of the following alkyl halides undergoes an E2 reaction:
a.
Problem 83b
Draw the major product obtained when each of the following alkyl halides undergoes an E2 reaction:
b.
Problem 83e
Draw the major product obtained when each of the following alkyl halides undergoes an E2 reaction:
e.
Problem 83f
Draw the major product obtained when each of the following alkyl halides undergoes an E2 reaction:
f.
Problem 84a,b
Draw the major product obtained when an alkyl halide in [PROBLEM 9-83] undergoes an E1 reaction.
a.
b.
Problem 84c
Draw the major product obtained when an alkyl halide in [PROBLEM 9-83] undergoes an E1 reaction.
c.
Problem 84d
Draw the major product obtained when an alkyl halide in [PROBLEM 9-83] undergoes an E1 reaction.
d.
Problem 85a
Indicate how each of the following factors affects an E1 reaction:
1. the strength of the base
2. the concentration of the base
3. the solvent
Problem 85b
Indicate how each of the same factors affects an E2 reaction.
1. the strength of the base
2. the concentration of the base
3. the solvent
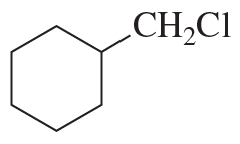
Problem 86a
Which species in each pair is more stable?
a.
Problem 86b
Which species in each pair is more stable?
b.
Problem 86c,d
Which species in each pair is more stable?
c.
d.
Problem 86e
Which species in each pair is more stable?
e.
Problem 86f
Which species in each pair is more stable?
f.
Problem 87
A chemist wanted to synthesize the anesthetic 2-ethoxy-2-methylpropane. He used ethoxide ion and 2-chloro-2-methylpropane for his synthesis and ended up with no ether. What was the product of his synthesis? What reagents should he have used?
Problem 88
Which reactant in each of the following pairs undergoes an elimination reaction more rapidly? Explain your choice.
a.
b.
Problem 89a,b
For each of the following reactions, draw the major elimination product; if the product can exist as stereoisomers, indicate which stereoisomer is obtained in greater yield.
a. (R)-2-bromohexane + high concentration of CH3O−
b. (R)-3-bromo-3-methylhexane + CH3OH
Problem 89c
For each of the following reactions, draw the major elimination product; if the product can exist as stereoisomers, indicate which stereoisomer is obtained in greater yield.
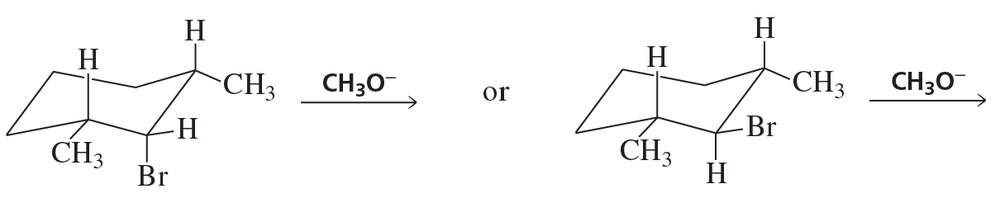
c. trans-1-chloro-2-methylcyclohexane + high concentration of CH3O−
Problem 89d
For each of the following reactions, draw the major elimination product; if the product can exist as stereoisomers, indicate which stereoisomer is obtained in greater yield.
d. trans-1-chloro-3-methylcyclohexane + high concentration of CH3O−
Problem 89e,f
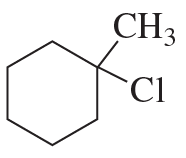
For each of the following reactions, draw the major elimination product; if the product can exist as stereoisomers, indicate which stereoisomer is obtained in greater yield.
e. 3-bromo-3-methylpentane + high concentration of CH3CH2O−
f. 3-bromo-3-methylpentane + CH3CH2OH
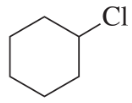
Problem 90
a. Which reacts faster in an E2 reaction: 3-bromocyclohexene or bromocyclohexane?
b. Which reacts faster in an E1 reaction?
Problem 91
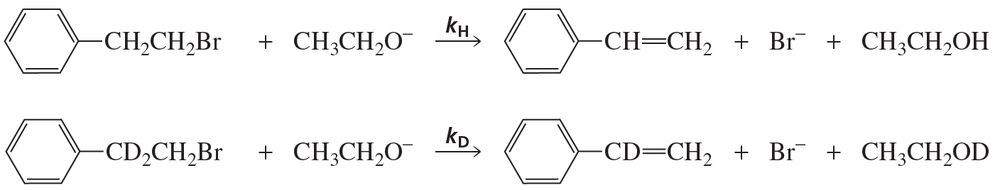
When the following reactions are carried out under the same conditions, the rate constant for the first reaction (kH) is found to be 7 times greater than the rate constant for the second reaction (kD). What does that tell you about the mechanism of the reaction? (Hint: a C—D bond is 1.2 kcal/mol stronger than a C—H bond.)
Problem 92a
Starting with an alkyl halide, how could the following compounds be prepared?
a. 2-methoxybutane
Problem 92b
Starting with an alkyl halide, how could the following compounds be prepared?
b. 1-methoxybutane