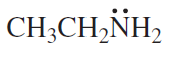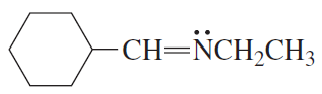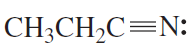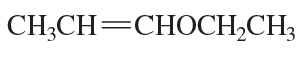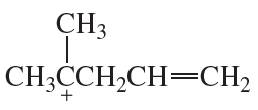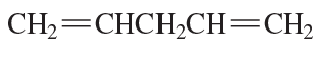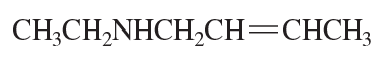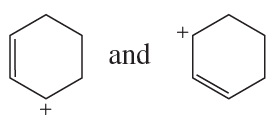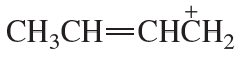Back
Back Bruice 8th Edition
Bruice 8th Edition Ch.8 - Delocalized Electrons:Their Effect on Stability, pKa, and the Products of a Reaction Aromaticity and Electronic Effects:An Introduction to the Reactions of Benzene
Ch.8 - Delocalized Electrons:Their Effect on Stability, pKa, and the Products of a Reaction Aromaticity and Electronic Effects:An Introduction to the Reactions of BenzeneProblem 55
Following the instructions for drawing the energy levels of the molecular orbitals for the compounds shown in [Figure 8.17], draw the energy levels of the molecular orbitals for the cycloheptatrienyl cation. For each compound, show the distribution of the electrons. Which of the compounds are aromatic?
Problem 55a
Following the instructions for drawing the energy levels of the molecular orbitals for the compounds shown in [Figure 8.17], draw the energy levels of the molecular orbitals for the cycloheptatrienyl cation. For each compound, show the distribution of the electrons. Which of the compounds are aromatic?
Problem 56
What orbital do the lone-pair electrons occupy in each of the following compounds?
a.
b.
c.
Problem 57a
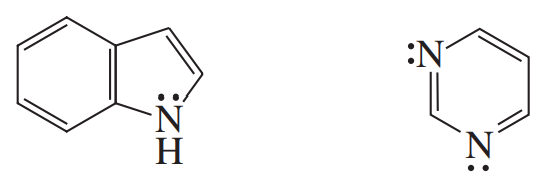
What orbitals contain the electrons represented as lone pairs in the structures of quinoline, indole, imidazole?
Problem 57b
What orbitals contain the electrons represented as lone pairs in the structures of purine, and pyrimidine?
Problem 59
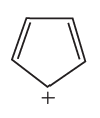
Which of the following compounds could be protonated without destroying its aromaticity?
Problem 60c
Refer to the electrostatic potential maps <IMAGE> to answer the following questions:
c. Why is the center of the electrostatic potential map of benzene more red than the center of the electrostatic potential map of pyridine?
Problem 61a,b,c
Which of the following has delocalized electrons?
a.
b.
c.
Problem 61g,h,i
Which of the following has delocalized electrons?
g.
h.
i.
Problem 61j,k,l
Which of the following has delocalized electrons?
j.
k.
l.
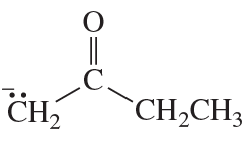
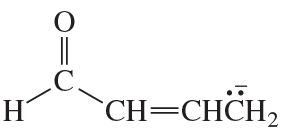
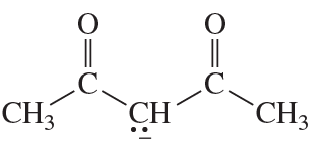
Problem 62(3)
a. Draw resonance contributors for the following species, showing all the lone pairs:
b. For each species, indicate the most stable resonance contributor.
3. NO2-
Problem 64a
Draw resonance contributors for the following ions:
a.
Problem 64d
Draw resonance contributors for the following ions:
d.
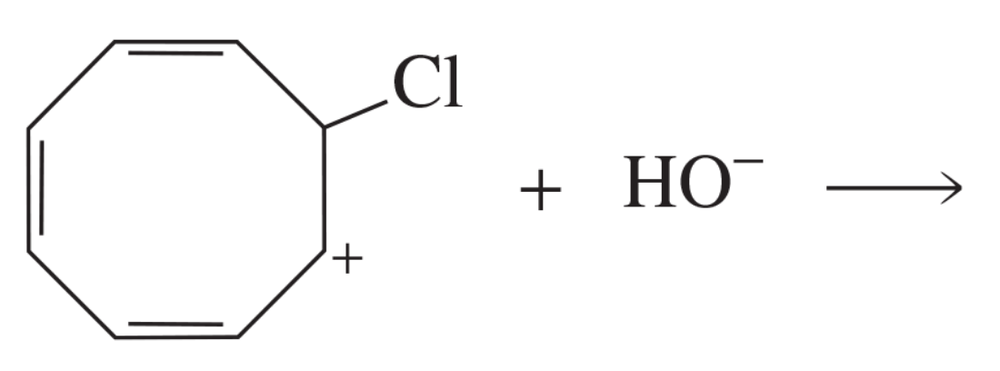
Problem 65
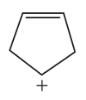
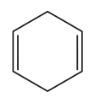
Draw all the products of the following reaction:
Problem 66d,e
Are the following pairs of structures resonance contributors or different compounds?
d.
e.
Problem 67a,b
a. How many linear dienes have molecular formula C6H10? (Disregard cis–trans isomers.)
b. How many of the linear dienes in part a are conjugated dienes?
Problem 68(10)
a. Draw resonance contributors for the following species. Do not include structures that are so unstable that their contributions to the resonance hybrid would be negligible. Indicate which are major contributors and which are minor contributors to the resonance hybrid.
b. Do any of the species have resonance contributors that all contribute equally to the resonance hybrid?
10.
Problem 68(5)
a. Draw resonance contributors for the following species. Do not include structures that are so unstable that their contributions to the resonance hybrid would be negligible. Indicate which are major contributors and which are minor contributors to the resonance hybrid.
b. Do any of the species have resonance contributors that all contribute equally to the resonance hybrid?
5.

Problem 68(7)
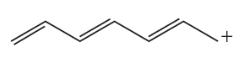
a. Draw resonance contributors for the following species. Do not include structures that are so unstable that their contributions to the resonance hybrid would be negligible. Indicate which are major contributors and which are minor contributors to the resonance hybrid.
b. Do any of the species have resonance contributors that all contribute equally to the resonance hybrid?
7.
Problem 68(9)
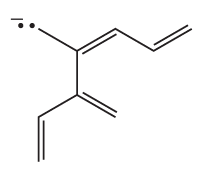
a. Draw resonance contributors for the following species. Do not include structures that are so unstable that their contributions to the resonance hybrid would be negligible. Indicate which are major contributors and which are minor contributors to the resonance hybrid.
b. Do any of the species have resonance contributors that all contribute equally to the resonance hybrid?
9.
Problem 68(14)
a. Draw resonance contributors for the following species. Do not include structures that are so unstable that their contributions to the resonance hybrid would be negligible. Indicate which are major contributors and which are minor contributors to the resonance hybrid.
b. Do any of the species have resonance contributors that all contribute equally to the resonance hybrid?
14.
Problem 68(6)
a. Draw resonance contributors for the following species. Do not include structures that are so unstable that their contributions to the resonance hybrid would be negligible. Indicate which are major contributors and which are minor contributors to the resonance hybrid.
b. Do any of the species have resonance contributors that all contribute equally to the resonance hybrid?
6.
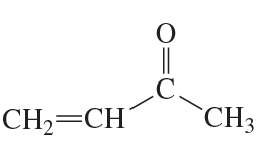
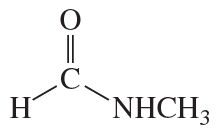
Problem 68(1)
a. Draw resonance contributors for the following species. Do not include structures that are so unstable that their contributions to the resonance hybrid would be negligible. Indicate which are major contributors and which are minor contributors to the resonance hybrid.
b. Do any of the species have resonance contributors that all contribute equally to the resonance hybrid?
- CH3CH=CHOCH3
Problem 68(3)
a. Draw resonance contributors for the following species. Do not include structures that are so unstable that their contributions to the resonance hybrid would be negligible. Indicate which are major contributors and which are minor contributors to the resonance hybrid.
b. Do any of the species have resonance contributors that all contribute equally to the resonance hybrid?
3.
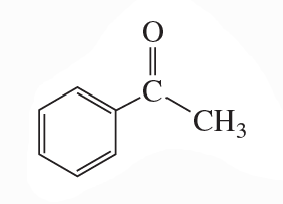
Problem 68(8)
a. Draw resonance contributors for the following species. Do not include structures that are so unstable that their contributions to the resonance hybrid would be negligible. Indicate which are major contributors and which are minor contributors to the resonance hybrid.
b. Do any of the species have resonance contributors that all contribute equally to the resonance hybrid?
8.
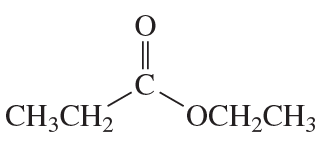
Problem 68(12)
a. Draw resonance contributors for the following species. Do not include structures that are so unstable that their contributions to the resonance hybrid would be negligible. Indicate which are major contributors and which are minor contributors to the resonance hybrid.
b. Do any of the species have resonance contributors that all contribute equally to the resonance hybrid?
12.
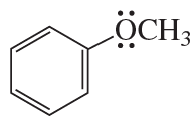
Problem 68(13)
a. Draw resonance contributors for the following species. Do not include structures that are so unstable that their contributions to the resonance hybrid would be negligible. Indicate which are major contributors and which are minor contributors to the resonance hybrid.
b. Do any of the species have resonance contributors that all contribute equally to the resonance hybrid?
13.
Problem 68(15)
a. Draw resonance contributors for the following species. Do not include structures that are so unstable that their contributions to the resonance hybrid would be negligible. Indicate which are major contributors and which are minor contributors to the resonance hybrid.
b. Do any of the species have resonance contributors that all contribute equally to the resonance hybrid?
15.
Problem 69
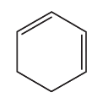
Which compound would you expect to have the greater heat of hydrogenation: 1,2-pentadiene or 1,4-pentadiene?
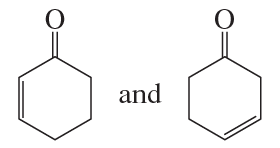
Problem 69a
Which ion in each of the following pairs is more stable?
a.