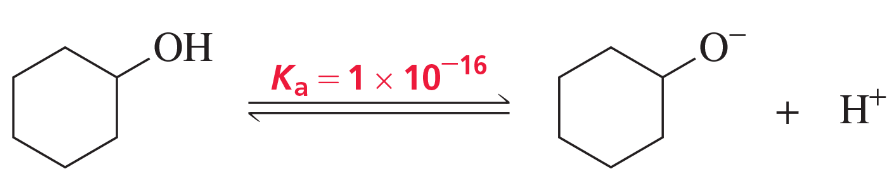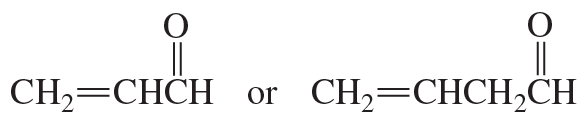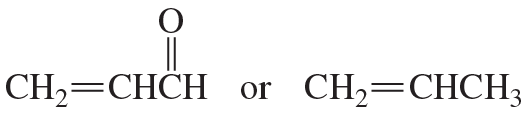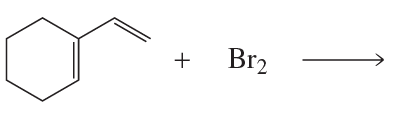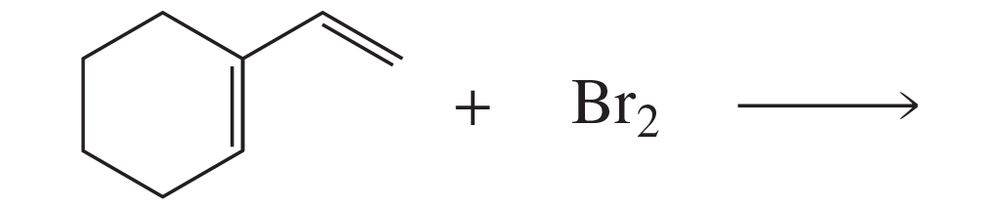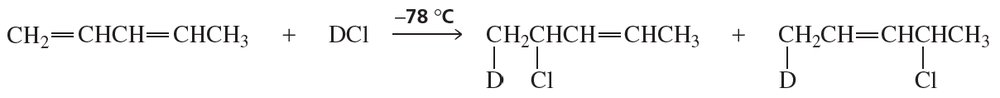Back
Back Bruice 8th Edition
Bruice 8th Edition Ch.8 - Delocalized Electrons:Their Effect on Stability, pKa, and the Products of a Reaction Aromaticity and Electronic Effects:An Introduction to the Reactions of Benzene
Ch.8 - Delocalized Electrons:Their Effect on Stability, pKa, and the Products of a Reaction Aromaticity and Electronic Effects:An Introduction to the Reactions of BenzeneProblem 90(2)
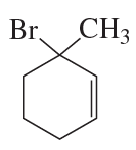
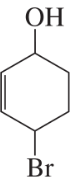
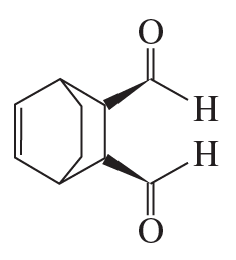
a. How could each of the following compounds be prepared from a hydrocarbon in a single step?
b. What other organic compound would be obtained from each synthesis?
2.
Problem 90(1)
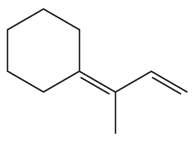
a. How could each of the following compounds be prepared from a hydrocarbon in a single step?
b. What other organic compound would be obtained from each synthesis?
1.
Problem 91
Draw the products obtained from the reaction of one equivalent of HBr with one equivalent of 1,3,5-hexatriene.
a. Which product(s) will predominate if the reaction is under kinetic control?
b. Which product(s) will predominate if the reaction is under thermodynamic control?
Problem 92a
How would the following substituents affect the rate of a Diels–Alder reaction?
a. an electron-donating substituent in the diene
Problem 92b
How would the following substituents affect the rate of a Diels–Alder reaction?
b. an electron-donating substituent in the dienophile
Problem 92c
How would the following substituents affect the rate of a Diels–Alder reaction?
c. an electron-withdrawing substituent in the diene
Problem 94a
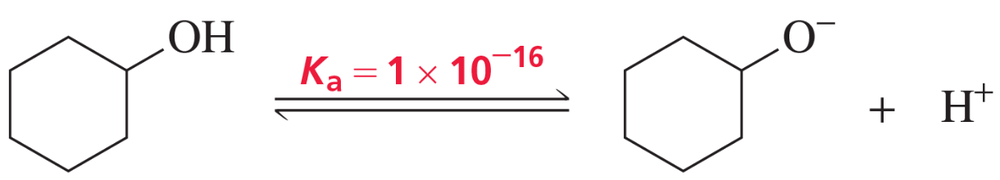
The acid dissociation constant (Ka) for loss of a proton from cyclohexanol is 1 × 10–16.
a. Draw a reaction coordinate diagram for loss of a proton from cyclohexanol.
Problem 94b
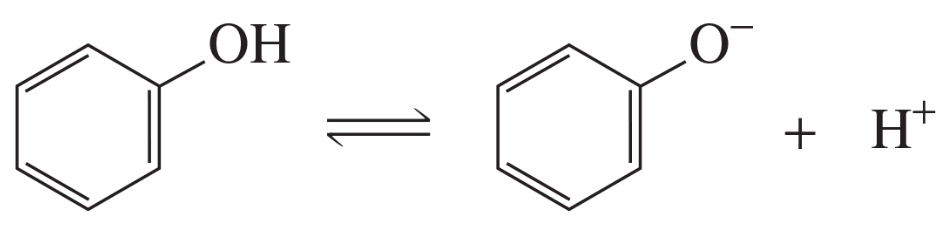
Draw the resonance contributors for phenol.
Problem 94c
Draw the resonance contributors for the phenolate ion.
Problem 94e,f
e. Which has a greater Ka: cyclohexanol or phenol?
f. Which is a stronger acid: cyclohexanol or phenol?
Problem 95e,f
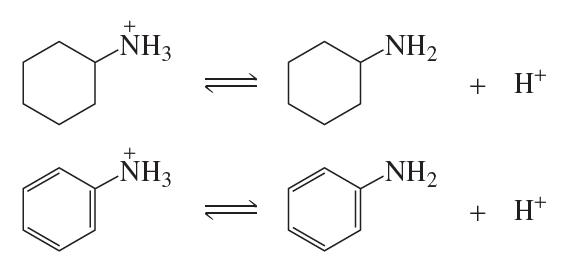
Protonated cyclohexylamine has a Ka = 1 * 10-11. Using the same sequence of steps as in Problem 94, determine which is a stronger base: cyclohexylamine
or aniline.
e. Which has a greater Ka: cyclohexylammmonium ion or anilinium ion?
f. Which is a stronger acid: cyclohexylamine or aniline?
Problem 98a(1)
Which dienophile in each pair is more reactive in a Diels–Alder reaction?
1.
Problem 98a(2)
Which dienophile in each pair is more reactive in a Diels–Alder reaction?
2.
Problem 99b
Draw the major products obtained from the reaction of one equivalent of HBr with the following compounds. For each reaction, indicate the kinetic product and the thermodynamic product.
b.
Problem 100
Cyclopentadiene can react with itself in a Diels–Alder reaction. Draw the endo and exo products.
Problem 101e
Which diene and which dienophile could be used to prepare each of the following?
e.
Problem 102a
Propose a mechanism for the following reaction:
Problem 102b
What is the product of the following reaction?
Problem 103a
What are the products of the following reaction?
Problem 103b
How many stereoisomers of each product could be obtained?
Problem 104
As many as 18 different Diels–Alder products can be obtained by heating a mixture of 1,3-butadiene and 2-methyl-1,3-butadiene. Identify the products.
Problem 105
On a single graph, draw the reaction coordinate diagram for the addition of one equivalent of HBr to 2-methyl-1,3-pentadiene and for the addition of one equivalent of HBr to 2-methyl-1,4-pentadiene. Which reaction is faster?
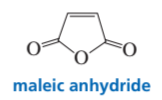
Problem 106
While attempting to recrystallize maleic anhydride, a student dissolved it in freshly distilled cyclopentadiene rather than in freshly distilled cyclopentane. Was her recrystallization successful?
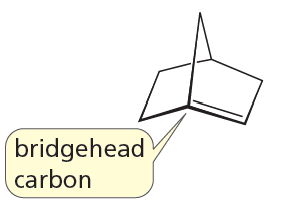
Problem 108
In 1935, J. Bredt, a German chemist, proposed that a bicycloalkene could not have a double bond at a bridgehead carbon unless one of the rings contains at least eight carbons. This is known as Bredt's rule. Explain why there cannot be a double bond at this position.
Problem 109a
The experiment shown next and discussed in Section 8.13 shows that the proximity of the chloride ion to C-2 in the transition state causes the 1,2-addition product to form more rapidly than the 1,4-addition product.
a. Why was it important for the investigators to know that the preceding reaction was being carried out under kinetic control?
Problem 110
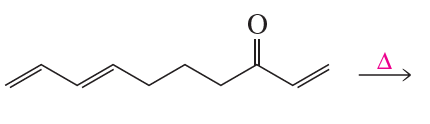
Identify the product of the following reaction.
Problem 111a
Draw the resonance contributors of the cyclooctatrienyl dianion.
a. Which of the resonance contributors is the least stable?
Problem 111b
Draw the resonance contributors of the cyclooctatrienyl dianion.
b. Which of the resonance contributors makes the smallest contribution to the hybrid?