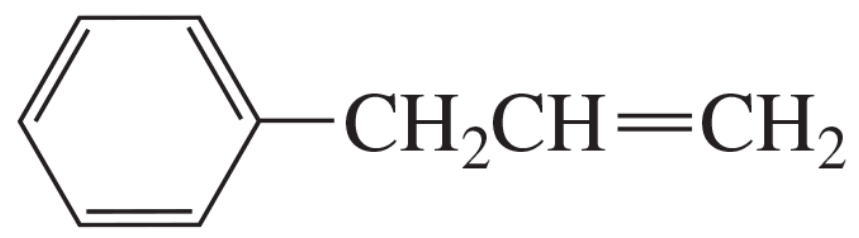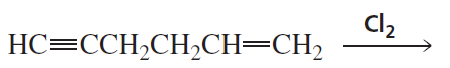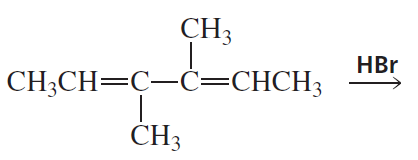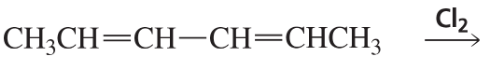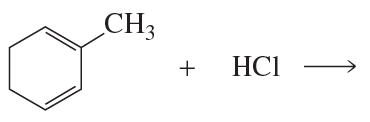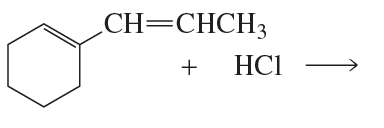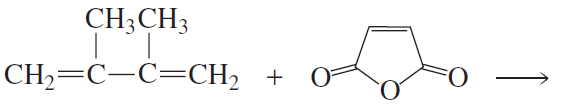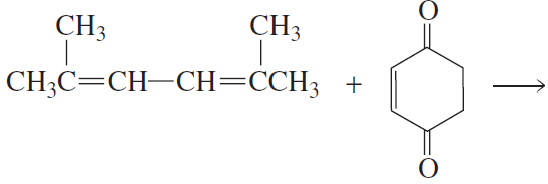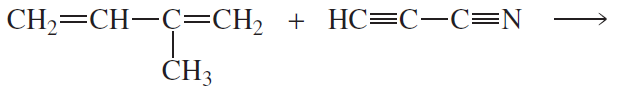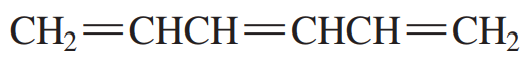Back
Back Bruice 8th Edition
Bruice 8th Edition Ch.8 - Delocalized Electrons:Their Effect on Stability, pKa, and the Products of a Reaction Aromaticity and Electronic Effects:An Introduction to the Reactions of Benzene
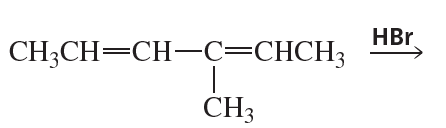
Ch.8 - Delocalized Electrons:Their Effect on Stability, pKa, and the Products of a Reaction Aromaticity and Electronic Effects:An Introduction to the Reactions of BenzeneProblem 24
What is the major product obtained from the addition of HBr to the following compound?
Problem 27b
What is the major product of each of the following reactions, assuming that one equivalent of each reagent is used in each reaction?
b.
Problem 27c
What is the major product of each of the following reactions, assuming that one equivalent of each reagent is used in each reaction?
c.
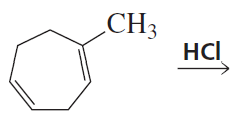
Problem 28b
What are the products of the following reactions, assuming that one equivalent of each reagent is used in each reaction?
b.
Problem 28c
What are the products of the following reactions, assuming that one equivalent of each reagent is used in each reaction?
c.
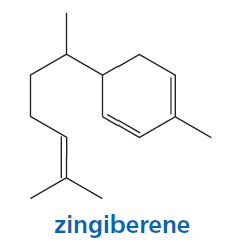
Problem 29
Which of the double bonds in zingiberene, the compound responsible for the aroma of ginger, is most reactive in an electrophilic addition reaction with HBr?
Problem 31
What products would be obtained from the reaction of 1,3,5-hexatriene with one equivalent of HBr? Disregard stereoisomers.
Problem 32a
What are the products of the following reactions, assuming that one equivalent of each reagent is used in each reaction? Disregard stereoisomers.
a.
Problem 32b
What are the products of the following reactions, assuming that one equivalent of each reagent is used in each reaction? Disregard stereoisomers.
b.
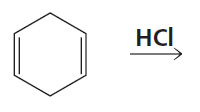
Problem 34
A student wanted to know whether the greater proximity of the nucleophile to the C-2 carbon in the transition state is what causes the 1,2-addition product to be formed faster when 1,3-butadiene reacts with HCl. Therefore, she decided to investigate the reaction of 2-methyl-1,3-cyclohexadiene with HCl. Her friend told her that she should use 1-methyl-1,3-cyclohexadiene instead. Should she follow her friend's advice?
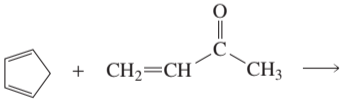
Problem 37a
What are the major 1,2- and 1,4-addition products of the following reaction? Indicate the kinetic and the thermodynamic products.
a.
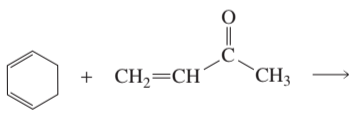
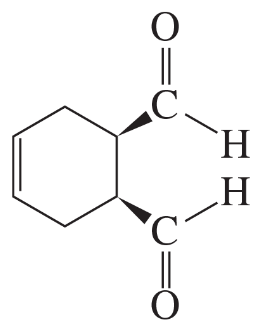
Problem 37b
What are the major 1,2- and 1,4-addition products of the following reaction? Indicate the kinetic and the thermodynamic products.
b.
Problem 39c
What are the products of the following reactions?
c.
Problem 39d
What are the products of the following reactions?
d.
Problem 40
What is the major product when the methoxy substituent in the preceding reaction is bonded to C-2 of the diene rather than to C-1?
Problem 41
Write a general rule that can be used to predict the major product of a Diels–Alder reaction between an alkene with an electron-withdrawing substituent and a diene with a substituent that can donate electrons by resonance depending on the location of the substituent on the diene.
Problem 42b
What two products are formed from each of the following reactions?
b.
Problem 44a
What are the products of the following reactions?
a.
Problem 44b
What are the products of the following reactions?
b.
Problem 46a
Explain why the following compounds are not optically active:
a. the product obtained from the reaction of 1,3-butadiene with cis-1,2-dichloroethene
Problem 46b
Explain why the following compounds are not optically active:
b. the product obtained from the reaction of 1,3-butadiene with trans-1,2-dichloroethene
Problem 47e
What diene and what dienophile should be used to synthesize the following?
e.
Problem 47f
What diene and what dienophile should be used to synthesize the following?
f.
Problem 48a
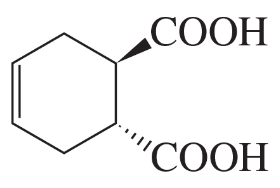
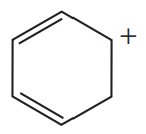
Which of the following are aromatic?
a.
b.
c.
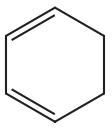
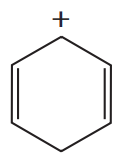
Problem 48b
Which of the following are aromatic?
d.
e.
f.
Problem 49a
What is the value of n in Hückel's rule when a compound has nine pairs of electrons? b. Is such a compound aromatic?
Problem 50a
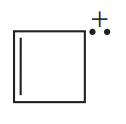
Which compound in each set is aromatic? Explain your choice.
a.
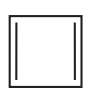
Problem 50b
Which compound in each set is aromatic? Explain your choice.
b.
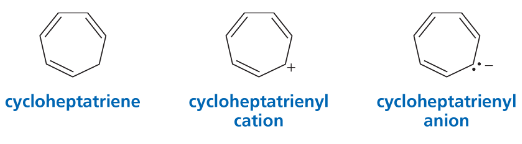
Problem 52a
Predict the relative pKa values of cyclopentadiene and cycloheptatriene.
Problem 52b
Predict the relative pKa values of cyclopropene and cyclopropane.