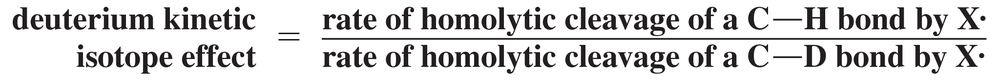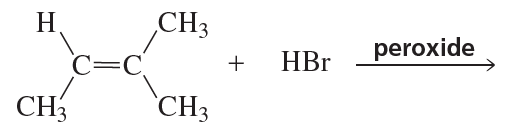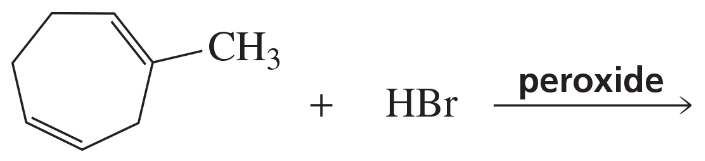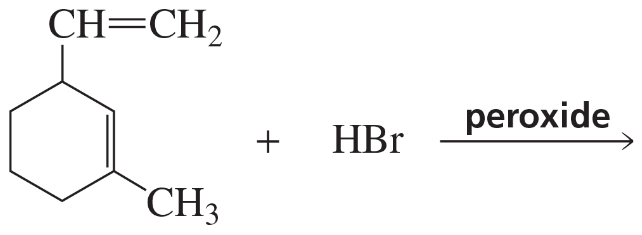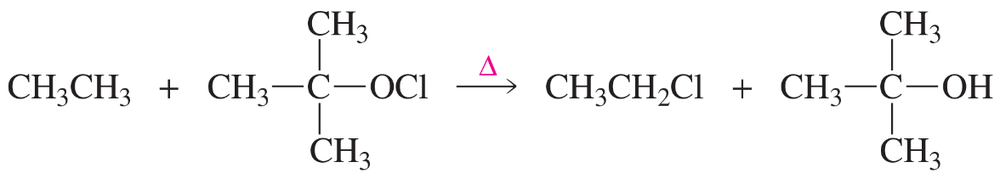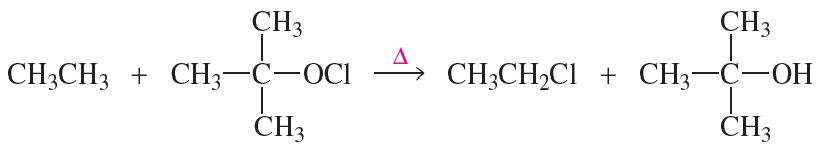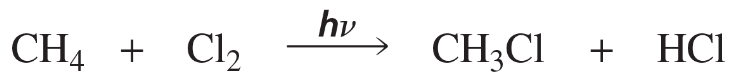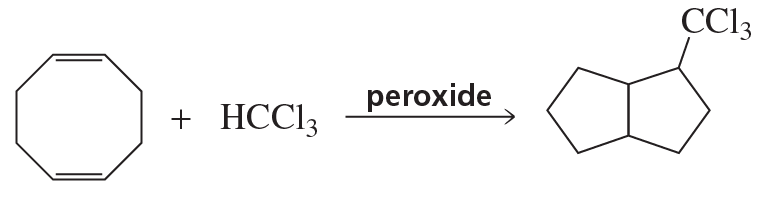Back
BackProblem 34
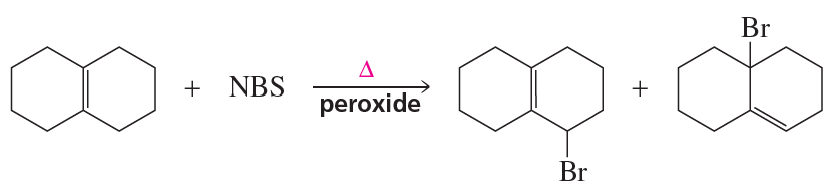
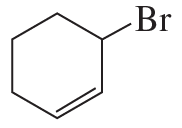
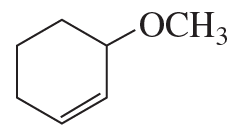
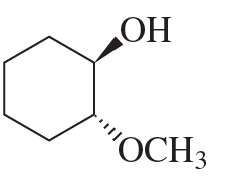
Propose a mechanism to account for the products formed in the following reaction:
Problem 35
The deuterium kinetic isotope effect for the halogenation of an alkane is defined in the following equation, where X・ = Cl・ or Br・
Predict whether chlorination or bromination would have a greater deuterium kinetic isotope effect.
Problem 37a
How many monochlorination products can be obtained from the radical chlorination of methylcyclohexane? Disregard stereoisomers.
Problem 37b
Which product would be obtained in greatest yield? Explain.
Problem 37c
How many monochlorination products would be obtained if all stereoisomers are included?
Problem 38a
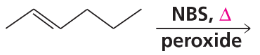
What five-carbon alkene forms the same product whether it reacts with HBr in the presence of a peroxide or with HBr in the absence of a peroxide?
Problem 38b
Draw the structures of four six-carbon alkenes that form the same product, whether they react with HBr in the presence of a peroxide or with HBr in the absence of a peroxide.
Problem 39a
What alkyl halide will be obtained in greatest yield? Ignore stereoisomers.
a.
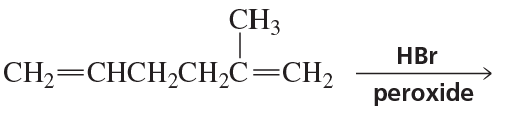
Problem 39b
What alkyl halide will be obtained in greatest yield? Ignore stereoisomers.
b.
Problem 39c
What alkyl halide will be obtained in greatest yield? Ignore stereoisomers.
c.
Problem 39d
What alkyl halide will be obtained in greatest yield? Ignore stereoisomers.
d.
Problem 39e
What alkyl halide will be obtained in greatest yield? Ignore stereoisomers.
e.
Problem 39f
What alkyl halide will be obtained in greatest yield? Ignore stereoisomers.
f.
Problem 40b
Starting with cyclohexane, how could the following compounds be prepared?
b.
Problem 40c
Starting with cyclohexane, how could the following compounds be prepared?
c.
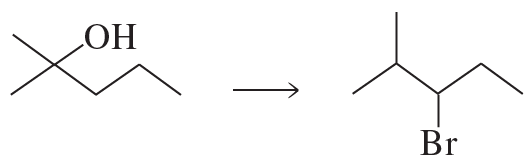
Problem 40e
Starting with cyclohexane, how could the following compounds be prepared?
e.
Problem 41a
Propose a mechanism for the following reaction:
Problem 41b
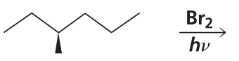
Given that ∆H° for the reaction is -42 kcal/mol and the bond dissociation enthalpies for the C−H, C−Cl, and O−H bonds are 101, 85, and 105 kcal/mol respectively, calculate the bond dissociation enthalpy of the O−Cl bond.
Problem 43a
Using the given starting material and any necessary organic or inorganic reagents, indicate how the desired compounds could be synthesized:
a.
Problem 43c
Using the given starting material and any necessary organic or inorganic reagents, indicate how the desired compounds could be synthesized:
c.
Problem 44
A chemist wanted to determine experimentally the relative ease of removing a hydrogen atom from a tertiary, a secondary, and a primary carbon by a chlorine radical. He allowed 2-methylbutane to undergo chlorination at 300 °C and obtained as products 36% 1-chloro-2-methylbutane, 18% 2-chloro-2-methylbutane, 28% 2-chloro-3-methylbutane, and 18% 1-chloro-3-methylbutane. What values did he obtain for the relative ease of removing a hydrogen atom from tertiary, secondary, and primary hydrogen carbons by a chlorine radical under the conditions of his experiment?
Problem 45
At 600 °C, the ratio of the relative rates of formation of a tertiary, a secondary, and a primary radical by a chlorine radical is 2.6 : 2.1 : 1. Explain the change in the degree of regioselectivity compared to what was found in Problem 44.
Problem 46c
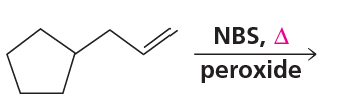
Draw the products of the following reactions, including all stereoisomers:
c.
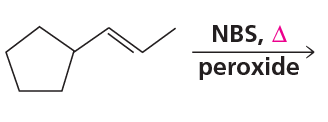
Problem 46d
Draw the products of the following reactions, including all stereoisomers:
d.
Problem 46e
Draw the products of the following reactions, including all stereoisomers:
e.
Problem 46f
Draw the products of the following reactions, including all stereoisomers:
f.
Problem 47a
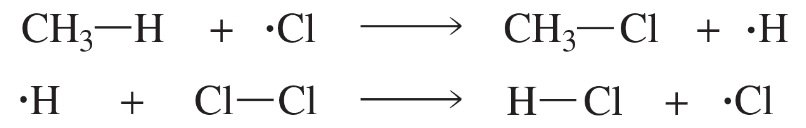
Calculate the ∆H° value for the following reaction:
Problem 48
A possible alternative mechanism to that shown in Problem 47 for the monochlorination of methane involves the following propagation steps:
How do you know that the reaction does not take place by this mechanism?
Problem 49
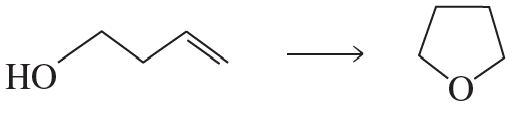
Propose a mechanism for the following reaction:
Problem 50
Explain why the rate of bromination of methane decreases if HBr is added to the reaction mixture.