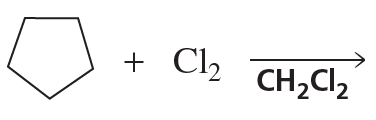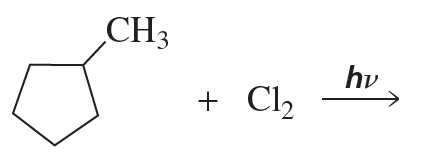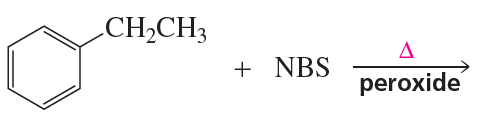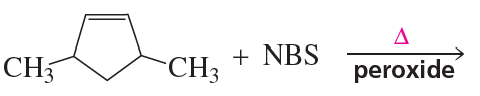Back
BackProblem 15a
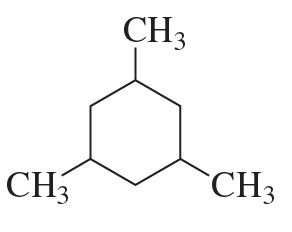
What hydrocarbon with molecular formula C4H10 forms only two monochlorinated products? Both products are achiral.
Problem 15b
What hydrocarbon with the same molecular formula as in part a forms three monochlorinated products? One is achiral and two are chiral.
Problem 18a
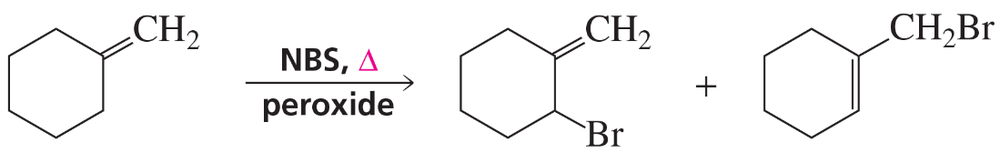
Two products are formed when methylenecyclohexane reacts with NBS? Show how each is formed. Disregard stereoisomers.
Problem 19
How many allylic substituted bromoalkenes are formed from the reactions in Problems 17 if stereoisomers are included?
Problem 19a
How many allylic substituted bromoalkenes are formed from the reactions in Problems 18 if stereoisomers are included?
Problem 20a
How many stereoisomers are formed from the reaction of cyclohexene with NBS?
Problem 20b
How many stereoisomers are formed from the reaction of 3-methylcyclohexene with NBS?
Problem 21a
Draw the resonance contributors for the following radicals:
a.
Problem 21c
Draw the resonance contributors for the following radicals:
c.
Problem 21d
Draw the resonance contributors for the following radicals:
d.
Problem 22a(3,4)
Draw the major product(s) of the reaction of 1-methylcyclohexene with the following reagents, disregarding stereoisomers:
3. HBr
4. HBr/peroxide
Problem 22a(1,2)
Draw the major product(s) of the reaction of 1-methylcyclohexene with the following reagents, disregarding stereoisomers:
1. NBS/∆/peroxide
2. Br2/CH2Cl2
Problem 22b(1,2)
For each reaction, show which stereoisomers are obtained
1. NBS/∆/peroxide
2. Br2/CH2Cl2
Problem 23a
Design a multistep synthesis to show how the following compounds can be prepared from the given starting material:
a.
Problem 23d
Design a multistep synthesis to show how the following compounds can be prepared from the given starting material:
d.
Problem 24
How many atoms share the unpaired electron in semiquinone?

Problem 26c
What are the product(s) of each of the following reactions? Disregard stereoisomers.
c.
Problem 26d
What are the product(s) of each of the following reactions? Disregard stereoisomers.
d.
Problem 26e
What are the product(s) of each of the following reactions? Disregard stereoisomers.
e.
Problem 26f
What are the product(s) of each of the following reactions? Disregard stereoisomers.
f.
Problem 27a
What alkane, with molecular formula C5H12, forms only one monochlorinated product when it is heated with Cl2?
Problem 28
Explain why iodine (I2) does not react with ethane, even though I2 is more easily cleaved homolytically than the other halogens.
Problem 29a
What is the major product obtained from treating an excess of each of the following compounds with Cl2 in the presence of ultraviolet light at room temperature? Disregard stereoisomers.
a.
Problem 29c
What is the major product obtained from treating an excess of each of the following compounds with Cl2 in the presence of ultraviolet light at room temperature? Disregard stereoisomers.
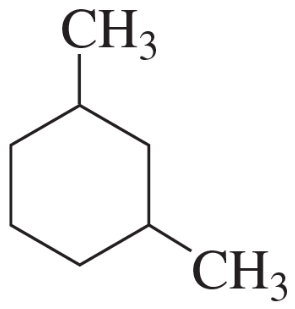
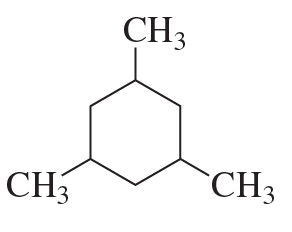
c.
Problem 30c
What are the answers to Problem 29 when the same compounds are treated with Br2 at 125 °C?
c.
Problem 31a
What is the major product of the following reactions? Disregard stereoisomers:
a.
Problem 31b
What is the major product of the following reactions? Disregard stereoisomers:
b.
Problem 31c
What is the major product of the following reactions? Disregard stereoisomers:
c.
Problem 31d
What is the major product of the following reactions? Disregard stereoisomers:
d.
Problem 32
When 2-methylpropane is monochlorinated in the presence of light at room temperature, 36% of the product is 2-chloro-2-methylpropane and 64% is 1-chloro-2-methylpropane. From these data, calculate how much easier it is to remove a hydrogen atom from a tertiary carbon than from a primary carbon under these conditions.