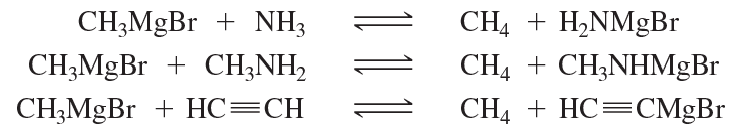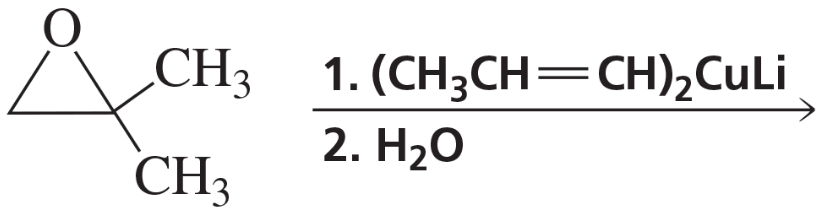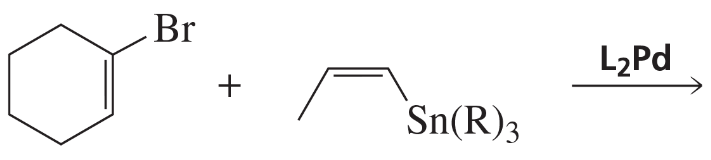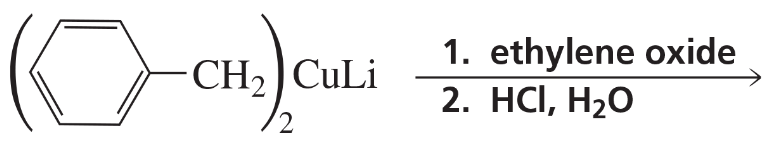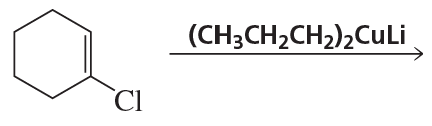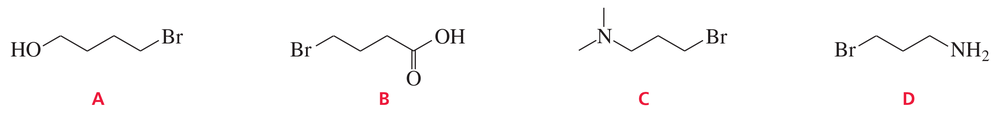Back
BackProblem 1a
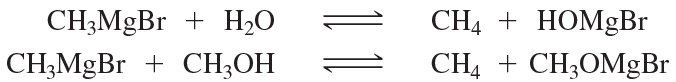
Which of the following reactions favor formation of the products? Recall that the equilibrium favors formation of the weaker acid.
Problem 1b
Which of the following reactions favor formation of the products? Recall that the equilibrium favors formation of the weaker acid.
Problem 2
Which is more reactive, an organolithium compound or an organosodium compound? Explain your answer.
Problem 3
What organometallic compound is formed from the reaction of excess methylmagnesium chloride and GaCl3?
Problem 5
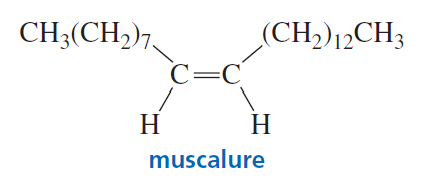
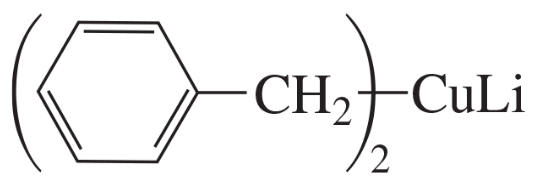
Muscalure is the sex attractant of the common housefly. Flies are lured to traps filled with bait that contain muscalure and an insecticide. Eating the bait is fatal. How could you synthesize muscalure using 1-bromopentane as one of the starting materials?
Problem 7b
What bromo-substituted compound would be required to react with (CH2=CH)2CuLi in order to form each of the following compounds?
b.
Problem 7c
What bromo-substituted compound would be required to react with (CH2=CH)2CuLi in order to form each of the following compounds?
c.
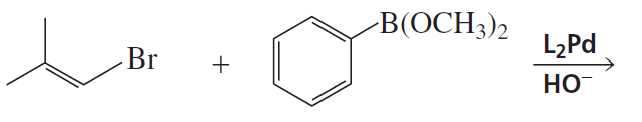
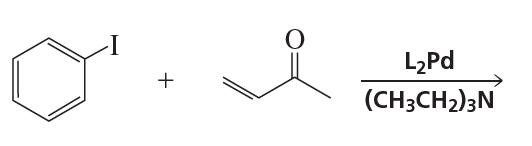
Problem 8b
What alcohols are formed from the reaction of ethylene oxide with the following organocuprates followed by the addition of acid?
b. (CH3CH=CH)2CuLi
Problem 8c
What alcohols are formed from the reaction of ethylene oxide with the following organocuprates followed by the addition of acid?
c.
Problem 9a
What are the products of the following reactions?
a.
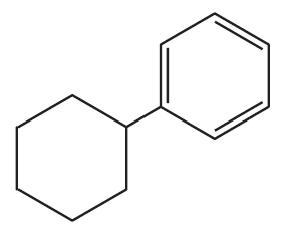
Problem 10a
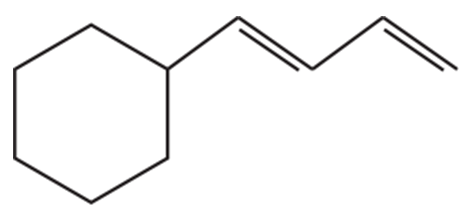
How could the following compounds be prepared, using cyclohexene as a starting material?
a.
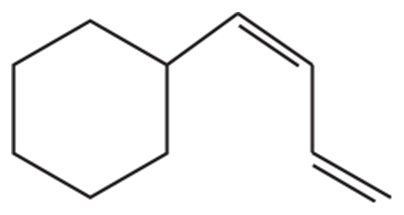
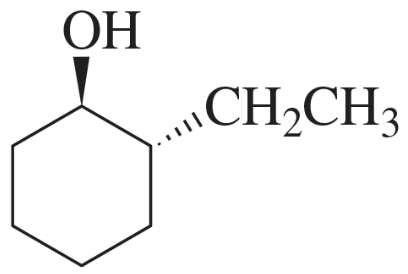
Problem 10d
How could the following compounds be prepared, using cyclohexene as a starting material?
d.
Problem 13a
What is the product of each of the following reactions?
a.
Problem 13b
What is the product of each of the following reactions?
b.
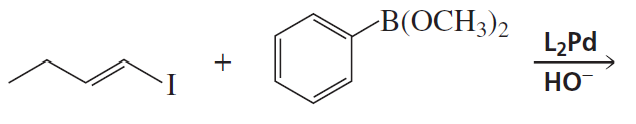
Problem 14c
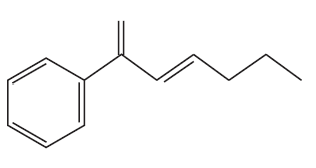
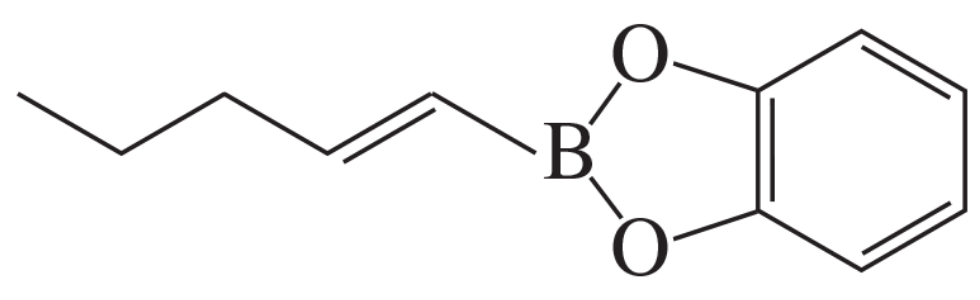
What aryl or vinylic halides would you use to synthesize the following compounds, using the alkenylorganoboron compound shown here?
c.
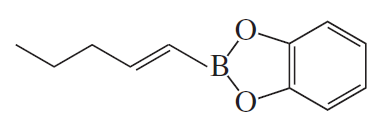
Problem 15
What hydrocarbon would you use to prepare the organoboron compound in Problem 14?
Problem 16a
The Stille reaction is similar to the Suzuki reaction. It replaces the alkenyl-organoboron compound of the Suzuki reaction with an alkenyl-organotin compound. (R is an alkyl group such as methyl or butyl.) Unlike the alkenyl-organoboron compound that always has a trans configuration, the alkenyl-organotin compound can have a cis configuration. What is the product of the Stille reaction shown here?
Problem 17a
What is the product of each of the following reactions?
a.
Problem 17b
What is the product of each of the following reactions?
b.
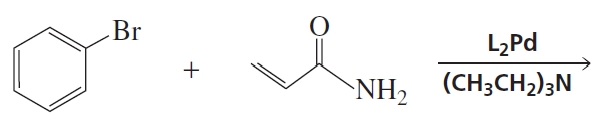
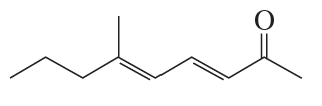
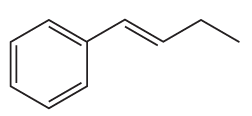
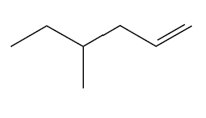
Problem 19a
What reactants are needed to synthesize each of the following compounds using a Heck reaction?
a.
Problem 20a
Show how the Suzuki and/or Heck reactions can be used to prepare the following compounds:
a.
Problem 22a
What products are obtained from metathesis of each of the following alkenes?
a. CH3CH2CH=CH2
Problem 22b
What products are obtained from metathesis of each of the following alkenes?
b.
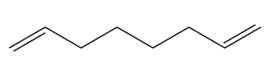
Problem 23a
Draw the product of ring-closing metathesis for each of the following compounds:
a.
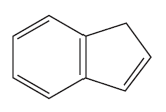
Problem 25a
What compound undergoes metathesis to form each of the following compounds?
a.
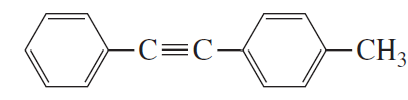
Problem 26
What new products are obtained from metathesis of the following alkyne?
Problem 27b
What are the products of the following reactions?
b.
Problem 27d
What are the products of the following reactions?
d.
Problem 27e
What are the products of the following reactions?
e.
Problem 28
Which of the following alkyl halides could be successfully used to form a Grignard reagent?