- 1. Introduction to Microbiology
- Introduction to Microbiology
- Introduction to Taxonomy
- Scientific Naming of Organisms
- Members of the Bacterial World
- Introduction to Bacteria
- Introduction to Archaea
- Introduction to Eukarya
- Acellular Infectious Agents: Viruses, Viroids & Prions
- Importance of Microorganisms
- Scientific Method
- Experimental Design
- 2. Disproving Spontaneous Generation
- 3. Chemical Principles of Microbiology
- 4. Water
- 5. Molecules of Microbiology
- 6. Cell Membrane & Transport
- Cell Envelope & Biological Membranes
- Bacterial & Eukaryotic Cell Membranes
- Archaeal Cell Membranes
- Types of Membrane Proteins
- Concentration Gradients and Diffusion
- Introduction to Membrane Transport
- Passive vs. Active Transport
- Osmosis
- Simple and Facilitated Diffusion
- Active Transport
- ABC Transporters
- Group Translocation
- Types of Small Molecule Transport Review
- Endocytosis and Exocytosis
- 7. Prokaryotic Cell Structures & Functions
- Prokaryotic & Eukaryotic Cells
- Binary Fission
- Generation Times
- Bacterial Cell Morphology & Arrangements
- Overview of Prokaryotic Cell Structure
- Introduction to Bacterial Cell Walls
- Gram-Positive Cell Walls
- Gram-Negative Cell Walls
- Gram-Positive vs. Gram-Negative Cell Walls
- The Glycocalyx: Capsules & Slime Layers
- Introduction to Biofilms
- Pili
- Fimbriae & Hami
- Introduction to Prokaryotic Flagella
- Prokaryotic Flagellar Structure
- Prokaryotic Flagellar Movement
- Proton Motive Force Drives Flagellar Motility
- Chemotaxis
- Review of Prokaryotic Surface Structures
- Prokaryotic Ribosomes
- Introduction to Bacterial Plasmids
- Cell Inclusions
- Endospores
- Sporulation
- Germination
- 8. Eukaryotic Cell Structures & Functions
- 9. Microscopes
- Introduction to Microscopes
- Magnification, Resolution, & Contrast
- Introduction to Light Microscopy
- Light Microscopy: Bright-Field Microscopes
- Light Microscopes that Increase Contrast
- Light Microscopes that Detect Fluorescence
- Electron Microscopes
- Reviewing the Different Types of Microscopes
- Introduction to Staining
- Simple Staining
- Differential Staining
- Other Types of Staining
- Reviewing the Types of Staining
- Gram Stain
- 10. Dynamics of Microbial Growth
- Biofilms
- Growing a Pure Culture
- Microbial Growth Curves in a Closed System
- Temperature Requirements for Microbial Growth
- Oxygen Requirements for Microbial Growth
- pH Requirements for Microbial Growth
- Osmolarity Factors for Microbial Growth
- Reviewing the Environmental Factors of Microbial Growth
- Nutritional Factors of Microbial Growth
- Growth Factors
- Introduction to Cultivating Microbial Growth
- Types of Solid Culture Media
- Plating Methods
- Measuring Growth by Direct Cell Counts
- Measuring Growth by Plate Counts
- Measuring Growth by Membrane Filtration
- Measuring Growth by Biomass
- Introduction to the Types of Culture Media
- Chemically Defined Media
- Complex Media
- Selective Media
- Differential Media
- Reducing Media
- Enrichment Media
- Reviewing the Types of Culture Media
- 11. Controlling Microbial Growth
- Introduction to Controlling Microbial Growth
- Selecting a Method to Control Microbial Growth
- Physical Methods to Control Microbial Growth
- Review of Physical Methods to Control Microbial Growth
- Chemical Methods to Control Microbial Growth
- Chemicals Used to Control Microbial Growth
- Liquid Chemicals: Alcohols, Aldehydes, & Biguanides
- Liquid Chemicals: Halogens
- Liquid Chemicals: Surface-Active Agents
- Other Types of Liquid Chemicals
- Chemical Gases: Ethylene Oxide, Ozone, & Formaldehyde
- Review of Chemicals Used to Control Microbial Growth
- Chemical Preservation of Perishable Products
- 12. Microbial Metabolism
- Introduction to Energy
- Laws of Thermodynamics
- Chemical Reactions
- ATP
- Enzymes
- Enzyme Activation Energy
- Enzyme Binding Factors
- Enzyme Inhibition
- Introduction to Metabolism
- Negative & Positive Feedback
- Redox Reactions
- Introduction to Aerobic Cellular Respiration
- Types of Phosphorylation
- Glycolysis
- Entner-Doudoroff Pathway
- Pentose-Phosphate Pathway
- Pyruvate Oxidation
- Krebs Cycle
- Electron Transport Chain
- Chemiosmosis
- Review of Aerobic Cellular Respiration
- Fermentation & Anaerobic Respiration
- 13. Photosynthesis
- 14. DNA Replication
- 15. Central Dogma & Gene Regulation
- Central Dogma
- Introduction to Transcription
- Steps of Transcription
- Transcription Termination in Prokaryotes
- Eukaryotic RNA Processing and Splicing
- Introduction to Types of RNA
- Genetic Code
- Introduction to Translation
- Steps of Translation
- Review of Transcription vs. Translation
- Prokaryotic Gene Expression
- Review of Prokaryotic vs. Eukaryotic Gene Expression
- Introduction to Regulation of Gene Expression
- Prokaryotic Gene Regulation via Operons
- The Lac Operon
- Glucose's Impact on Lac Operon
- The Trp Operon
- Review of the Lac Operon & Trp Operon
- Introduction to Eukaryotic Gene Regulation
- Eukaryotic Chromatin Modifications
- Eukaryotic Transcriptional Control
- Eukaryotic Post-Transcriptional Regulation
- Post-Translational Modification
- Eukaryotic Post-Translational Regulation
- 16. Microbial Genetics
- Introduction to Microbial Genetics
- Introduction to Mutations
- Methods of Inducing Mutations
- Prototrophs vs. Auxotrophs
- Mutant Detection
- The Ames Test
- Introduction to DNA Repair
- DNA Repair Mechanisms
- Horizontal Gene Transfer
- Bacterial Transformation
- Transduction
- Introduction to Conjugation
- Conjugation: F Plasmids
- Conjugation: Hfr & F' Cells
- Genome Variability
- CRISPR CAS
- 17. Biotechnology
- 18. Viruses, Viroids, & Prions
- Introduction to Viruses
- Introduction to Bacteriophage Infections
- Bacteriophage: Lytic Phage Infections
- Bacteriophage: Lysogenic Phage Infections
- Bacteriophage: Filamentous Phage Infections
- Plaque Assays
- Introduction to Animal Virus Infections
- Animal Viruses: 1. Attachment to the Host Cell
- Animal Viruses: 2. Entry & Uncoating in the Host Cell
- Animal Viruses: 3. Synthesis & Replication
- Animal Viruses: DNA Virus Synthesis & Replication
- Animal Viruses: RNA Virus Synthesis & Replication
- Animal Viruses: Antigenic Drift vs. Antigenic Shift
- Animal Viruses: Reverse-Transcribing Virus Synthesis & Replication
- Animal Viruses: 4. Assembly Inside Host Cell
- Animal Viruses: 5. Release from Host Cell
- Acute vs. Persistent Viral Infections
- COVID-19 (SARS-CoV-2)
- Plant Viruses
- Viroids
- Prions
- 19. Innate Immunity
- Introduction to Immunity
- Introduction to Innate Immunity
- Introduction to First-Line Defenses
- Physical Barriers in First-Line Defenses: Skin
- Physical Barriers in First-Line Defenses: Mucous Membrane
- First-Line Defenses: Chemical Barriers
- First-Line Defenses: Normal Microflora
- Introduction to Cells of the Immune System
- Cells of the Immune System: Granulocytes
- Cells of the Immune System: Agranulocytes
- Introduction to Cell Communication
- Cell Communication: Surface Receptors & Adhesion Molecules
- Cell Communication: Cytokines
- Pattern Recognition Receptors (PRRs)
- Introduction to the Complement System
- Activation Pathways of the Complement System
- Effects of the Complement System
- Review of the Complement System
- Phagoctytosis
- Introduction to Inflammation
- Steps of the Inflammatory Response
- Fever
- Interferon Response
- 20. Adaptive Immunity
- Introduction to Adaptive Immunity
- Antigens
- Introduction to T Lymphocytes
- Major Histocompatibility Complex Molecules
- Activation of T Lymphocytes
- Functions of T Lymphocytes
- Review of Cytotoxic vs Helper T Cells
- Introduction to B Lymphocytes
- Antibodies
- Classes of Antibodies
- Outcomes of Antibody Binding to Antigen
- T Dependent & T Independent Antigens
- Clonal Selection
- Antibody Class Switching
- Affinity Maturation
- Primary and Secondary Response of Adaptive Immunity
- Immune Tolerance
- Regulatory T Cells
- Natural Killer Cells
- Review of Adaptive Immunity
- 21. Principles of Disease
- Symbiotic Relationships
- The Human Microbiome
- Characteristics of Infectious Disease
- Stages of Infectious Disease Progression
- Koch's Postulates
- Molecular Koch's Postulates
- Bacterial Pathogenesis
- Introduction to Pathogenic Toxins
- Exotoxins Cause Damage to the Host
- Endotoxin Causes Damage to the Host
- Exotoxins vs. Endotoxin Review
- Immune Response Damage to the Host
- Introduction to Avoiding Host Defense Mechanisms
- 1) Hide Within Host Cells
- 2) Avoiding Phagocytosis
- 3) Surviving Inside Phagocytic Cells
- 4) Avoiding Complement System
- 5) Avoiding Antibodies
- Viruses Evade the Immune Response
- 25. Epidemiology
- 26. Applications of the Immune Response
- 27. Immunological Disorders
- 28. Antimicrobial Drugs
- Introduction to Antimicrobial Drugs
- How Antimicrobial Drugs Work
- Broad vs Narrow Spectrum Drugs
- Superinfections
- Drug Interactions: Synergism and Antagonism
- Therapeutic Window & Therapeutic Index
- Inhibitors of Cell Wall Synthesis: Beta-lactam & Penicillin
- Inhibitors of Cell Wall Synthesis: Polypeptide Antibiotics & Isoniazid
- Inhibitors of Protein Synthesis
- Disruptors of Cell Membranes
- Inhibitors of Nucleic Acid Synthesis
- Competitive Inhibitors of Metabolic Pathways
- Antifungal Drugs
- Antiviral Drugs
- Tests to Guide Antimicrobial Use
- Antimicrobial Resistance
- 29. Microbial Infections - Skin and Eyes
- 30. Microbial Infections - Respiratory System
- 31. Microbial Infections - Digestive System
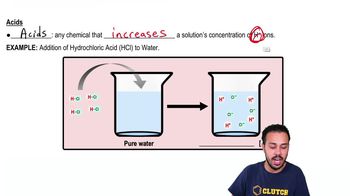
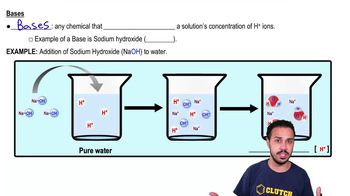
4. Water
Acids and Bases
Practice this topic
- Multiple Choice
Which of the following reactions is most consistent with that of a base?
a) NH4+ → NH3 + H+
b) H2CO3 → HCO3- + H+
c) NaOH → Na+ + OH-
d) HCl → H+ + Cl-
- Multiple Choice
The addition of an acid like HCl to an aqueous solution (pure water) would result in:
a) An increase in pH only.
b) Both the release of H+ and an increase in pH.
c) Both the release of H+ and a decrease in pH.
d) The release of H+ into the solution only.
e) A decrease in pH only.
- Multiple Choice
In what way(s) do bases work to increase the pH of a solution?
a) Increasing the concentration of hydroxide ions.
b) Decreasing the concentration of hydrogen ions.
c) Decreasing the concentration of hydroxide ions.
d) Increasing the concentration of hydrogen ions.
e) Both a & b.
f) Both c & d.
- Open Question
Classify each of the molecules on the left as an acid, base, or salt. The dissociation products of the molecules are shown to help you.
HNO₃ →H⁺ + NO⁻₃
- Open Question
Classify each of the molecules on the left as an acid, base, or salt. The dissociation products of the molecules are shown to help you.
H₂SO₄ →2H⁺ + SO²₄⁻
- Open Question
Classify each of the molecules on the left as an acid, base, or salt. The dissociation products of the molecules are shown to help you.
NaOH → Na⁺ + OH⁻
- Open Question
Classify each of the molecules on the left as an acid, base, or salt. The dissociation products of the molecules are shown to help you.
MgSO₄ → Mg²⁺ + SO²₄⁻