3. Chemical Principles of Microbiology
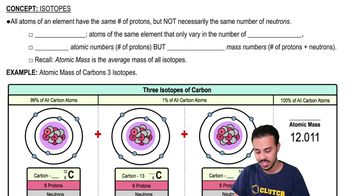
Isotopes
Practice this topic
- Multiple Choice
What is TRUE about carbon-13 and carbon-14?
a) They are isotopes.
b) They have the same mass number.
c) They have the same number of neutrons in their nuclei.
d) They behave differently in biological reactions.
e) None of the above are true.
- Multiple Choice
How are Carbon-13 and Nitrogen-15 respectively different from the more abundant isotopes Carbon-12 and Nitrogen-14? Carbon-13 and Nitrogen-15 _______________:
a) Each have an extra neutron.
b) Each have an extra proton.
c) Each have one less neutron.
d) Each have one less proton.
e) Each have one less electron.
- Multiple Choice
The atomic number of nitrogen is 7. Nitrogen-15 has a greater mass number than nitrogen-14 because the atomic nucleus of nitrogen-15 contains ________.
a) 7 neutrons.
b) 8 neutrons.
c) 8 protons.
d) 15 protons.
- Multiple Choice
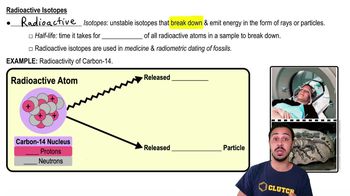
Radioactive isotopes are utilized for all of the following except:
a) Dating fossilized material of once living things.
b) Radiation treatment to slow or stop the development of cancer cells.
c) Labeling regions of the body with radioactivity for special imaging techniques.
d) All of the above.
- Open Question
Radioisotopes are frequently used to label molecules in a cell. The fate of atoms and molecules in a cell can then be followed. This process is the basis for questions 1-3.
Assume E. coli bacteria are grown in a nutrient medium containing the radioisotope ¹⁶N. After a 48-hour incubation period, the ¹⁶N would most likely be found in the E. coli’s
a. carbohydrates.
b. lipids.
c. proteins.
d. water.
e. none of the above
- Open Question
Radioisotopes are frequently used to label molecules in a cell. The fate of atoms and molecules in a cell can then be followed. This process is the basis for questions 1-3.
If Pseudomonas bacteria are supplied with radioactively labeled cytosine, after a 24-hour incubation period this cytosine would most likely be found in the cells’
a. carbohydrates.
b. DNA.
c. lipids.
d. water.
e. proteins.
- Open Question
Radioisotopes are frequently used to label molecules in a cell. The fate of atoms and molecules in a cell can then be followed. This process is the basis for questions 1-3.
If E. coli were grown in a medium containing the radioactive isotope ³²P, the ³²P would be found in all of the following molecules of the cell except
a. ATP.
b. carbohydrates.
c. DNA.
d. plasma membrane.
e. complex lipids.
- Open Question
Indicate the true statements and then correct the false statements so that they are true.
a. Isotopes are atoms with differing numbers of protons and the same number of
neutrons.
b. A cation is a positive ion.
c. Redox reactions create ions.
d. Equal sharing of electrons leads to polar covalent bonds.
e. Ions are charged atoms.
f. CO2 is an inorganic molecule.
g. Isomers have the same molecular formula but different structures.
h. Adding a base to a solution will decrease the pH.