4. Water
pH Scale
Practice this topic
- Multiple Choice
In a neutral solution, the concentration of __________.
a) Hydrogen ions is less than the concentration of hydroxide ions.
b) Water molecules is less than the concentration of hydroxide ions.
c) Hydrogen ions is greater than the concentration of hydroxide ions.
d) Hydrogen ions is equal to the concentration of hydroxide ions.
- Multiple Choice
A base _______:
a) Has a value of 7 on the pH scale.
b) Is a chemical that donates hydrogen ions to a solution.
c) Is a chemical that accepts hydrogen ions from a solution.
d) Has a value below 7 on the pH scale.
e) None of the above are correct.
- Multiple Choice
Which of the following statements about buffers is true?
a) They maintain a consistent pH only when acids are added to them, but not bases.
b) They maintain a consistent pH of 7.
c) They fluctuate in pH when acids are added to them.
d) They maintain a consistent pH when acids or bases are added to them.
e) They fluctuate in pH when acids or bases are added to them.
- Multiple Choice
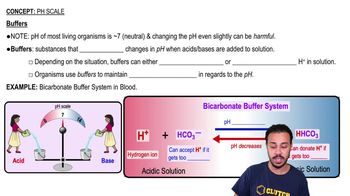
Buffers are substances that help resist shifts in pH by:
a) Donating H+ in acidic solutions.
b) Donating H+ to a solution when they have been depleted.
c) Releasing OH− in basic solutions.
d) Accepting H+ when they are in excess.
e) Both b and d are correct.
- Open Question
How many more times acidic is a solution with a pH of 9 versus a solution with a pH of 12?
- Open Question
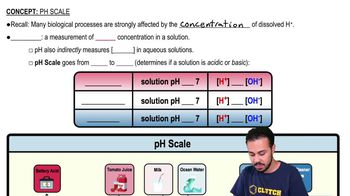
The __________ scale is a measure of the concentration of hydrogen ions in a solution.
- Open Question
Which of the following statements about a carbonated cola beverage with a pH of 2.9 is true?
a. It has a relatively high concentration of hydrogen ions.
b. It has a relatively low concentration of hydrogen ions.
c. It has equal amounts of hydroxyl and hydrogen ions.
d. Cola is a buffered solution.