3. Water
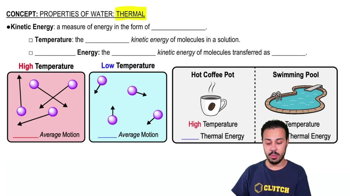
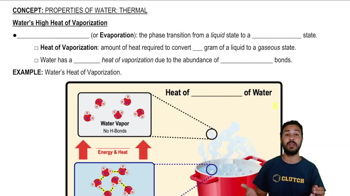
Properties of Water- Thermal
3. Water
Properties of Water- Thermal
Additional 2 creators.
Learn with other creators
Showing 5 of 5 videos
Practice this topic
- Multiple Choice
Which of the following is due to the high specific heat of water?
a) Oil does not mix with water.
b) A lake heats up more slowly than the surrounding environment.
c) The high surface tension of water.
d) Sugar dissolves in hot tea faster than in iced tea.
- Multiple Choice
Which if the following defines the term evaporation?
a) The conversion of a liquid into a vapor.
b) The conversion of a solid into a vapor.
c) The conversion of a vapor into a liquid.
d) The conversion of a vapor into a solid.
- Multiple Choice
Choose the correct statement: Liquid water ________.
a) Is less dense than ice.
b) Has a lower specific heat than most other molecules.
c) Has a higher heat of vaporization than most other molecules.
d) Is nonpolar.
- Multiple ChoiceWhich action would involve the greatest transfer of heat?
- Open QuestionWater has a high heat-absorbing capacity because .a. the sun's rays penetrate to the bottom of bodies of water, mainly heating the bottom surface;b. the strong covalent bonds that hold individual water molecules together require large inputs of heat to break;c. it has the ability to dissolve many heat-resistant solutes;d. initial energy inputs are first used to break hydrogen bonds between water molecules and only after these are broken, to raise the temperature;e. all of the above are true
- Open QuestionA slice of pizza has 500 kcal. If we could burn the pizza and use all the heat to warm a 50-L container of cold water, what would be the approximate increase in the temperature of the water? (Note: A liter of cold water weighs about 1 kg.)a. 50°Cb. 5°Cc. 100°Cd. 10°C
- Open QuestionFrom what you have learned about water, why do coastal regions tend to have milder climates with cooler summers and warmer winters than do inland areas at the same latitude?