3. Water
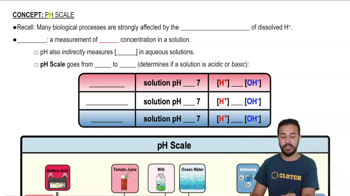
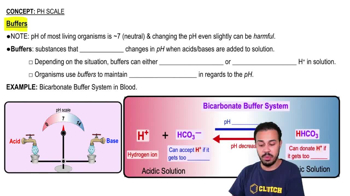
pH Scale
Learn with other creators
Practice this topic
- Multiple Choice
In a neutral solution, the concentration of __________.
a) Hydrogen ions is less than the concentration of hydroxide ions.
b) Water molecules is less than the concentration of hydroxide ions.
c) Hydrogen ions is greater than the concentration of hydroxide ions.
d) Hydrogen ions is equal to the concentration of hydroxide ions.
- Multiple Choice
A base _______:
a) Has a value of 7 on the pH scale.
b) Is a chemical that donates hydrogen ions to a solution.
c) Is a chemical that accepts hydrogen ions from a solution.
d) Has a value below 7 on the pH scale.
e) None of the above are correct.
- Multiple Choice
Which of the following statements about buffers is true?
a) They maintain a consistent pH only when acids are added to them, but not bases.
b) They maintain a consistent pH of 7.
c) They fluctuate in pH when acids are added to them.
d) They maintain a consistent pH when acids or bases are added to them.
e) They fluctuate in pH when acids or bases are added to them.
- Multiple ChoiceA substance that minimizes changes in the concentration of H+ and OH– in a solution is a __________.
- Open QuestionMeasurements show that the pH of a particular lake is 4.0. What is the hydrogen ion concentration of the lake?a. 4.0 Mb. 10−10Mc. 10−4Md. 104M
- Open QuestionWhat is the hydroxide ion concentration of the lake described in question 3?a. 10−10Mb. 10−4Mc. 10−7Md. 10.0 M
- Open QuestionA solution at pH 6 contains _________ H+ than the same amount of a solution at pH 8.a. 20 times moreb. 100 times morec. 2 times lessd. 100 times less
- Open QuestionA can of cola consists mostly of sugar dissolved in water, with some carbon dioxide gas that makes it fizzy and makes the pH less than 7. In chemical terms, you could say that cola is an aqueous solution where water is the _________ , sugar is a _________ , and carbon dioxide makes the solution _________ .a. solvent . . . solute . . . basicb. solute . . . solvent . . . basicc. solvent . . . solute . . . acidicd. solute . . . solvent . . . acidic