2. Chemistry
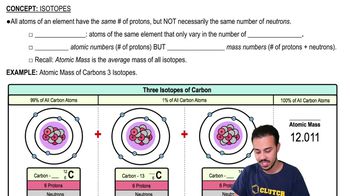
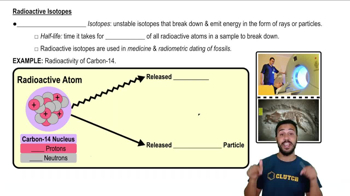
Isotopes
Learn with other creators
Practice this topic
- Multiple Choice
What is TRUE about carbon-13 and carbon-14?
a) They are isotopes.
b) They have the same mass number.
c) They have the same number of neutrons in their nuclei.
d) They behave differently in biological reactions.
e) None of the above are true.
- Multiple Choice
How are Carbon-13 and Nitrogen-15 respectively different from the more abundant isotopes Carbon-12 and Nitrogen-14? Carbon-13 and Nitrogen-15 _______________:
a) Each have an extra neutron.
b) Each have an extra proton.
c) Each have one less neutron.
d) Each have one less proton.
e) Each have one less electron.
- Multiple Choice
The atomic number of nitrogen is 7. Nitrogen-15 has a greater mass number than nitrogen-14 because the atomic nucleus of nitrogen-15 contains ________.
a) 7 neutrons.
b) 8 neutrons.
c) 8 protons.
d) 15 protons.
- Multiple Choice
Radioactive isotopes are utilized for all of the following except:
a) Dating fossilized material of once living things.
b) Radiation treatment to slow or stop the development of cancer cells.
c) Labeling regions of the body with radioactivity for special imaging techniques.
d) All of the above.
- Open QuestionWe can represent atoms by listing the number of protons, neutrons, and electrons—for example, 2p+,2n0,2e− for helium. Which of the following represents the 18O isotope of oxygen?a. 7p+,2n0,9e−b. 8p+,10n0,8e−c. 9p+,9n0,9e−d. 10p+,8n0,9e-1views