Back
BackProblem 1a
Draw three-dimensional representations of the following amino acids.
(a) L-phenylalanine
Problem 1b
Draw three-dimensional representations of the following amino acids.
(b) L-histidine
Problem 1c
Draw three-dimensional representations of the following amino acids.
(c) D-serine
Problem 1d
Draw three-dimensional representations of the following amino acids.
(d) L-tryptophan
Problem 2a
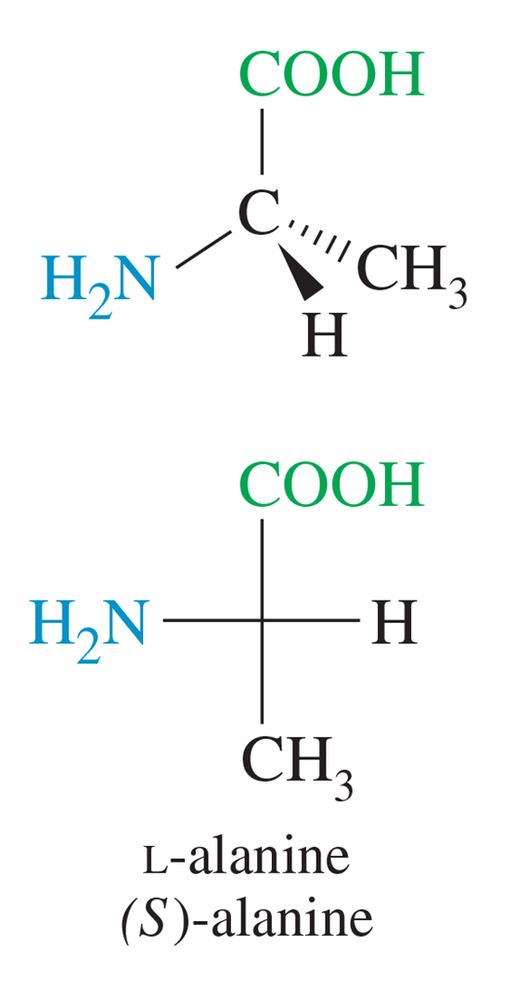
Most naturally occurring amino acids have chiral centers (the asymmetric α carbon atoms) that are named (S) by the Cahn–Ingold–Prelog convention (Section 5-3). The common naturally occurring form of cysteine has a chiral center that is named (R), however.
(a) What is the relationship between (R)-cysteine and (S)-alanine? Do they have the opposite three-dimensional configuration (as the names might suggest) or the same configuration?
Problem 2b
Most naturally occurring amino acids have chiral centers (the asymmetric α carbon atoms) that are named (S) by the Cahn–Ingold–Prelog convention (Section 5-3). The common naturally occurring form of cysteine has a chiral center that is named (R), however.
(b) (S)-Alanine is an L-amino acid (Figure 24-2). Is (R)-cysteine a d-amino acid or an L-amino acid?
Problem 3
The herbicide glyphosate (Roundup®) kills plants by inhibiting an enzyme needed for synthesis of phenylalanine. Deprived of phenylalanine, the plant cannot make the proteins it needs, and it gradually weakens and dies. Although a small amount of glyphosate is deadly to a plant, its human toxicity is quite low. Suggest why this powerful herbicide has little effect on humans.
Problem 4a
Draw the structure of the predominant form of
(a) isoleucine at pH 11.
Problem 4b
Draw the structure of the predominant form of
(b) proline at pH 2.
Problem 4d
Draw the structure of the predominant form of
(d) glutamic acid at pH 7.
Problem 4e(iii)
Draw the structure of the predominant form of
(e) a mixture of alanine, lysine, and aspartic acid at (iii) pH 2.
Problem 4e(i)
Draw the structure of the predominant form of
(e) a mixture of alanine, lysine, and aspartic acid at (i) pH 6;
Problem 6
Although tryptophan contains a heterocyclic amine, it is considered a neutral amino acid. Explain why the indole nitrogen of tryptophan is more weakly basic than one of the imidazole nitrogens of histidine.
Problem 7
Draw the electrophoretic separation of Ala, Lys, and Asp at pH 9.7.
Problem 8
Draw the electrophoretic separation of Trp, Cys, and His at pH 6.0.
Problem 9a
Show how the following amino acids might be formed in the laboratory by reductive amination of the appropriate α-ketoacid.
(a) alanine
Problem 10b
Show how you would use bromination followed by amination to synthesize the following amino acids.
(b) leucine
Problem 11a
Show how you would use a Strecker synthesis to make phenylalanine.
Problem 11b(1)
Propose a mechanism for each step in the synthesis in part (a).
Problem 11b(2)
Propose a mechanism for each step in the synthesis in part (a).
Problem 12b
Show how you would use a Strecker synthesis to make
(b) valine.
Problem 12c
Show how you would use a Strecker synthesis to make
(c) aspartic acid.
Problem 13
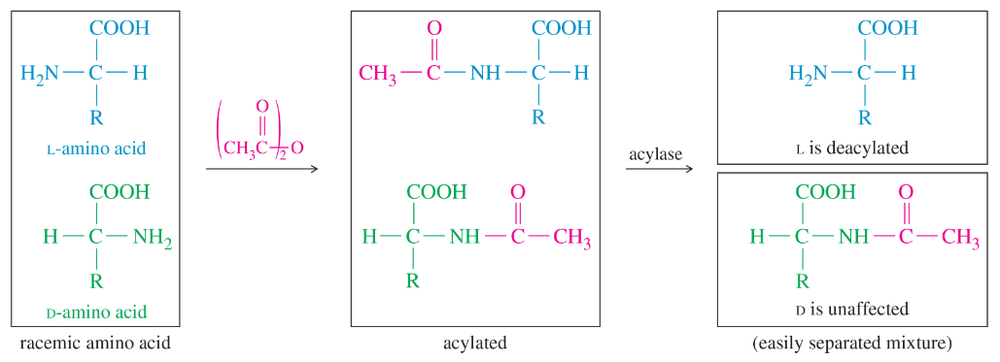
Suggest how you would separate the free L-amino acid from its acylated D enantiomer in Figure 24-5.
Problem 15
Give equations for the formation and hydrogenolysis of glutamine benzyl ester.
Problem 16
Give equations for the formation and hydrogenolysis of N-benzyloxycarbonyl methionine.
Problem 17
Use resonance forms to show delocalization of the negative charge in the Ruhemann's purple anion.
Problem 19a
Draw the structure of the phenylthiohydantoin derivatives of
(a) alanine.
Problem 19c
Draw the structure of the phenylthiohydantoin derivatives of
(c) lysine.
Problem 24a
Propose a mechanism for the coupling of acetic acid and aniline using DCC as a coupling agent.
Problem 27a
(a) The isoelectric point (pI) of phenylalanine is pH 5.5. Draw the structure of the major form of phenylalanine at pH values of 1, 5.5, and 11.