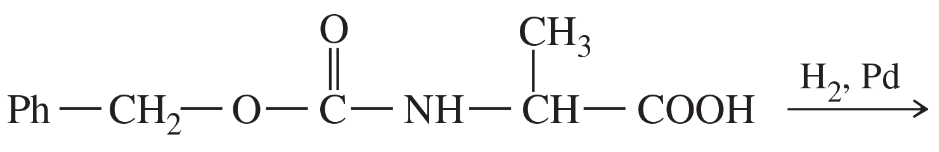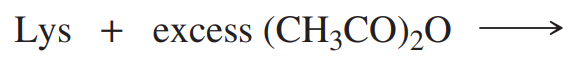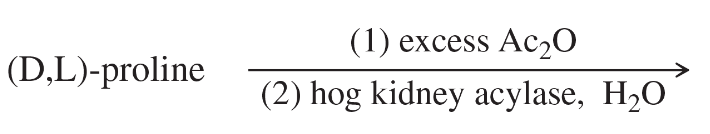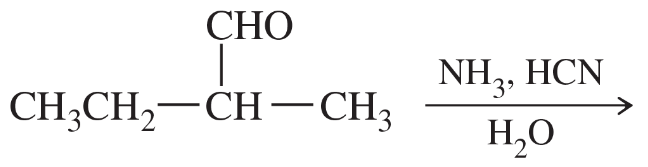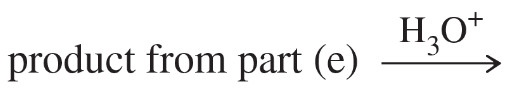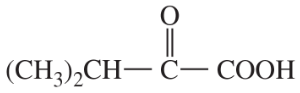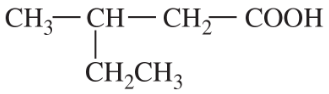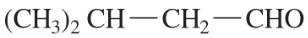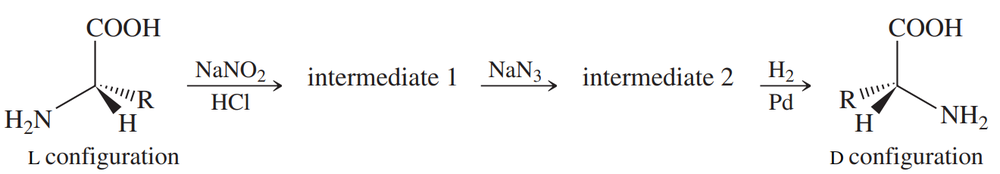Back
BackProblem 27c
The isoelectric point of glutamic acid is pH 3.2. Draw the structures of the major forms of glutamic acid at pH values of 1, 3.2, 7, and 11. Explain why the side-chain carboxylic acid is a weaker acid than the acid group next to the α-carbon atom.
Problem 29b
Predict the products of the following reactions.
(b)
Problem 29c
Predict the products of the following reactions.
(c)
Problem 29d
Predict the products of the following reactions.
(d)
Problem 29e
Predict the products of the following reactions.
(e)
Problem 29f
Predict the products of the following reactions.
(f)
Problem 29g
Predict the products of the following reactions.
(g) 4-methylpentanoic acid + Br2/PBr3 →
Problem 29h
Predict the products of the following reactions.
(h) product from part (g) + excess NH3 →
Problem 30a
Show how you would synthesize any of the standard amino acids from each starting material. You may use any necessary reagents.
(a)
Problem 30b
Show how you would synthesize any of the standard amino acids from each starting material. You may use any necessary reagents.
(b)
Problem 30c
Show how you would synthesize any of the standard amino acids from each starting material. You may use any necessary reagents.
(c)
Problem 31a,b
Show how you would convert alanine to the following derivatives. Show the structure of the product in each case.
(a) alanine isopropyl ester
(b) N-benzoylalanine
Problem 31c,d
Show how you would convert alanine to the following derivatives. Show the structure of the product in each case.
(c) N-benzyloxycarbonyl alanine
(d) tert-butyloxycarbonyl alanine
Problem 32
Suggest a method for the synthesis of the unnatural D enantiomer of alanine from the readily available L enantiomer of lactic acid.
Problem 33
Show how you would use the Strecker synthesis to make tryptophan. What stereochemistry would you expect in your synthetic product?
Problem 36
Aspartame (Nutrasweet®) is a remarkably sweet-tasting dipeptide ester. Complete hydrolysis of aspartame gives phenyl alanine, aspartic acid, and methanol. Mild incubation with carboxypeptidase has no effect on aspartame. Treatment of aspartame with phenyl isothiocyanate, followed by mild hydrolysis, gives the phenylthiohydantoin of aspartic acid. Propose a structure for aspartame.
Problem 40
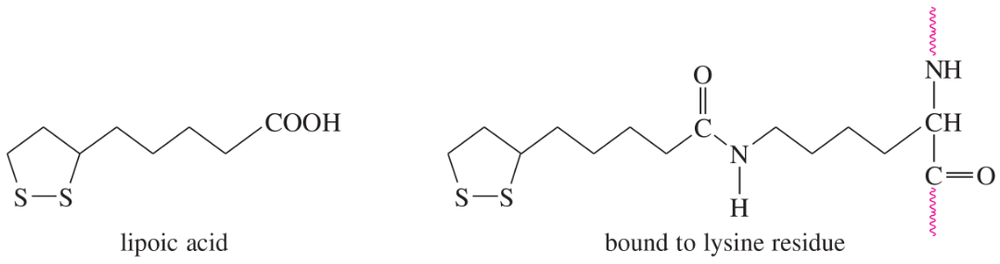
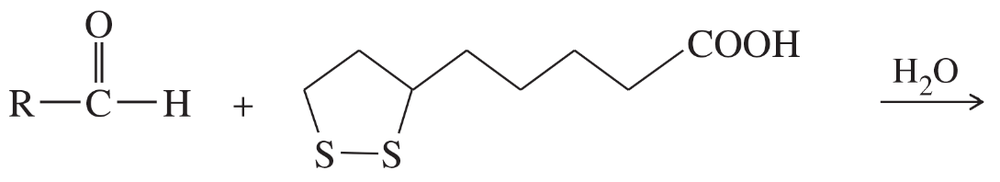
Lipoic acid is often found near the active sites of enzymes, usually bound to the peptide by a long, flexible amide linkage with a lysine residue.
(a) Is lipoic acid a mild oxidizing agent or a mild reducing agent? Draw it in both its oxidized and reduced forms.
(b) Show how lipoic acid might react with two Cys residues to form a disulfide bridge.
(c) Give a balanced equation for the hypothetical oxidation or reduction, as you predicted in part (a), of an aldehyde by lipoic acid.
Problem 41
Histidine is an important catalytic residue found at the active sites of many enzymes. In many cases, histidine appears to remove protons or to transfer protons from one location to another.
(a) Show which nitrogen atom of the histidine heterocycle is basic and which is not.
(b) Use resonance forms to show why the protonated form of histidine is a particularly stable cation.
(c) Show the structure that results when histidine accepts a proton on the basic nitrogen of the heterocycle and then is deprotonated on the other heterocyclic nitrogen. Explain how histidine might function as a pipeline to transfer protons between sites within an enzyme and its substrate.
Problem 42
Metabolism of arginine produces urea and the rare amino acid ornithine. Ornithine has an isoelectric point close to 10. Propose a structure for ornithine.
Problem 45a
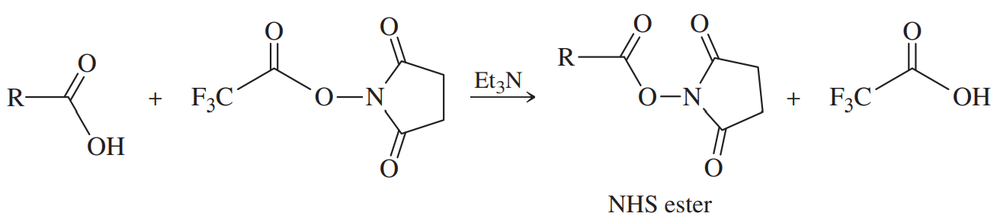
There are many methods for activating a carboxylic acid in preparation for coupling with an amine. The following method converts the acid to an N-hydroxysuccinimide (NHS) ester.
(a) Explain why an NHS ester is much more reactive than a simple alkyl ester.
Problem 45b
There are many methods for activating a carboxylic acid in preparation for coupling with an amine. The following method converts the acid to an N-hydroxysuccinimide (NHS) ester.
(b) Propose a mechanism for the reaction shown.
Problem 45c
There are many methods for activating a carboxylic acid in preparation for coupling with an amine. The following method converts the acid to an N-hydroxysuccinimide (NHS) ester.
(c) Propose a mechanism for the reaction of the NHS ester with an amine, R–NH2.
Problem 46
Sometimes chemists need the unnatural D enantiomer of an amino acid, often as part of a drug or an insecticide. Most L-amino acids are isolated from proteins, but the D-amino acids are rarely found in natural proteins. D-amino acids can be synthesized from the corresponding L-amino acids. The following synthetic scheme is one of the possible methods.
(a) Draw the structures of intermediates 1 and 2 in this scheme.
(b) How do we know that the product is entirely the unnatural D configuration?
Problem 48d,e
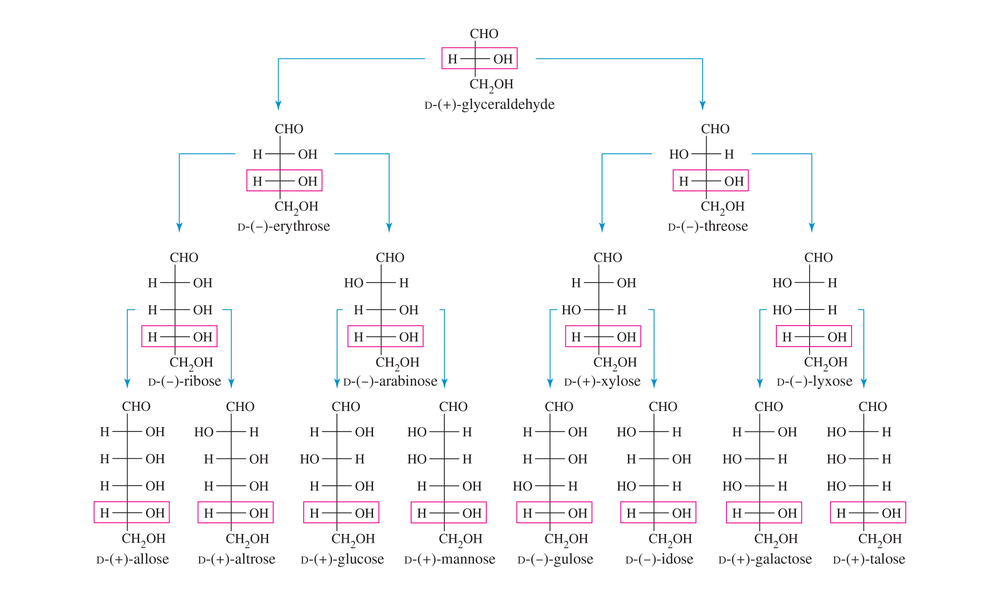
Use Figure 23-3 (the D family of aldoses) to name the following aldoses.
(d) the enantiomer of D-galactose
(e) the C5 epimer of D-glucose