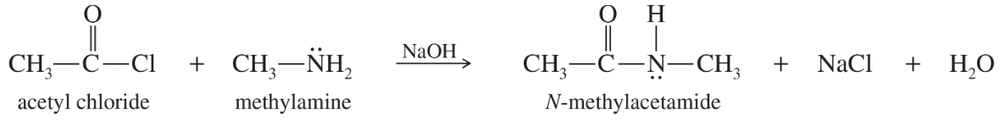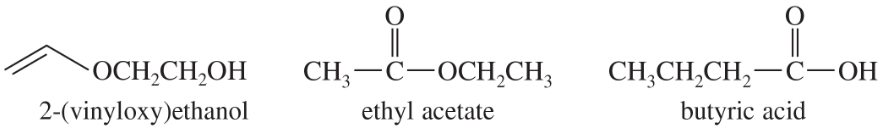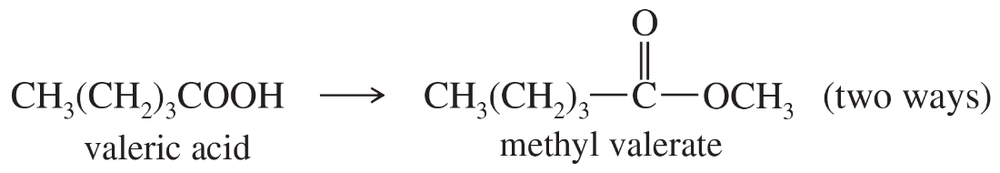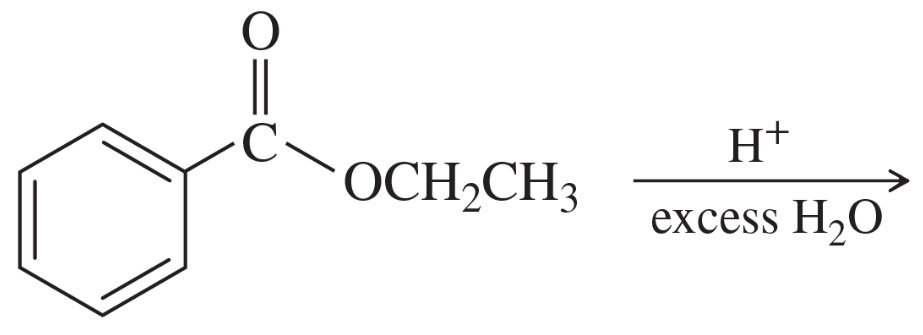Back
BackProblem 20a
Draw the following sugars using Haworth projections:
a. β-D-galactopyranose
Problem 20b
Draw the following sugars using Haworth projections:
b. α-D-tagatopyranose
Problem 21a1
Show how the following ketones might be synthesized from the indicated acids, using any necessary reagents.
(a) propiophenone from propionic acid (using alkylation of the acid)
Problem 21b
Show how the following ketones might be synthesized from the indicated acids, using any necessary reagents.
(b) methyl cyclohexyl ketone from cyclohexanecarboxylic acid
Problem 22
Propose a mechanism for the reaction of benzoic acid with oxalyl chloride. This mechanism begins like the thionyl chloride reaction, to give a reactive mixed anhydride. Nucleophilic acyl substitution by chloride ion gives a tetrahedral intermediate that eliminates a leaving group, which then fragments into carbon dioxide, carbon monoxide, and chloride ion.
Problem 23a
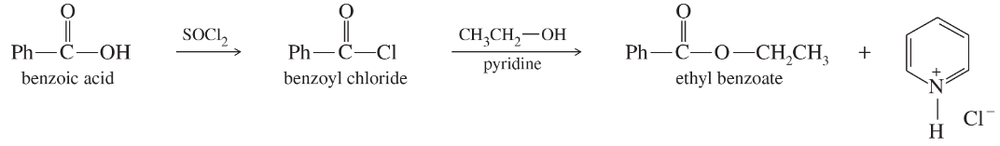
Propose mechanisms for the nucleophilic acyl substitutions to form ethyl benzoate as shown on the previous page.
Problem 23b
Propose mechanisms for the nucleophilic acyl substitutions to form N-methylacetamide as shown on the previous page.
Problem 24a
Show how you would use an acid chloride as an intermediate to synthesize.
(a) N-phenylbenzamide (PhCONHPh) from benzoic acid and aniline.
Problem 24b
Show how you would use an acid chloride as an intermediate to synthesize
(b) phenyl propionate (CH3CH2COOPh) from propionic acid and phenol.
Problem 25a,b
Give the IUPAC names of the following compounds.
(a) CH3CH2C≡CCOOH
(b) CH3CH(NH2)CH(OH)COOH
Problem 25c,d
Give the IUPAC names of the following compounds.
(c) (CH3)2C=CHCOOH
(d)
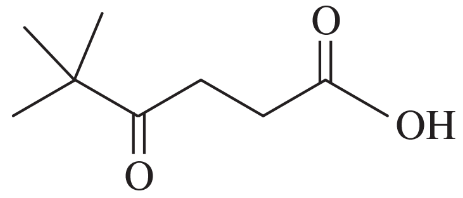
Problem 25e,f
Give the IUPAC names of the following compounds.
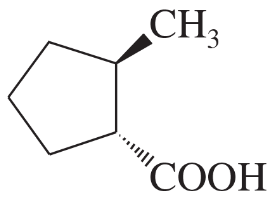
(e)
(f)
Problem 27f-i
Draw the structures of the following compounds.
(f) salicylic acid
(g) zinc undecanoate (athlete's-foot powder)
(h) sodium benzoate (a food preservative)
(i) sodium fluoroacetate (Compound 1080, a controversial coyote poison)
Problem 28
Show how you would use extractions with a separatory funnel to separate a mixture of the following compounds: benzoic acid, phenol, benzyl alcohol, aniline
Problem 29c,d
Arrange each group of compounds in order of increasing basicity.
c. sodium benzoate, sodium ethoxide, and sodium phenoxide
d. pyridine, sodium ethoxide, and sodium acetate
Problem 30a,b,c
Predict the products (if any) of the following acid–base reactions.
(a) acetic acid + ammonia
(b) phthalic acid + excess NaOH
(c) p-toluic acid + potassium trifluoroacetate
Problem 30d,e
Predict the products (if any) of the following acid–base reactions.
(d) α-bromopropionic acid + sodium propionate
(e) benzoic acid + sodium phenoxide
Problem 31
Rank the following isomers in order of increasing boiling point, and explain the reasons for your order of ranking.
Problem 32
Arrange each group of compounds in order of increasing acidity.
(a) phenol, ethanol, acetic acid
(b) p-toluenesulfonic acid, acetic acid, chloroacetic acid
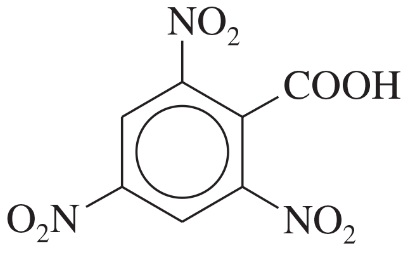
(c) benzoic acid, o-nitrobenzoic acid, m-nitrobenzoic acid
(d) butyric acid, α-bromobutyric acid, β-bromobutyric acid
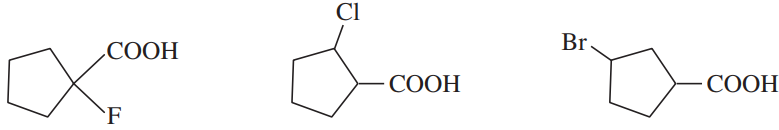
(e)
Problem 34a,b
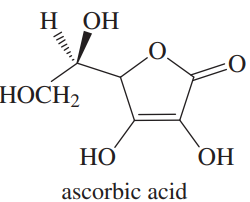
Given the structure of ascorbic acid (vitamin C):
(a) Is ascorbic acid a carboxylic acid?
(b) Compare the acid strength of ascorbic acid (pKa = 4.71) with acetic acid.
Problem 34c,d
Given the structure of ascorbic acid (vitamin C):
(c) Predict which proton in ascorbic acid is the most acidic.
(d) Draw the form of ascorbic acid that is present in the body (aqueous solution, pH = 7.4)
Problem 36a
Show how you would accomplish the following syntheses efficiently (you may use any necessary reagents).
(a) trans-1-bromobut-2-ene → trans-pent-3-enoic acid (two ways)
Problem 36b
Show how you would accomplish the following syntheses efficiently (you may use any necessary reagents).
(b) hex-3-ene → propanoic acid
Problem 36c
Show how you would accomplish the following syntheses efficiently (you may use any necessary reagents).
(c) but-2-enal → but-2-enoic acid
Problem 36d
Show how you would accomplish the following syntheses efficiently (you may use any necessary reagents).
(d) hexanoic acid → hexanal
Problem 36e
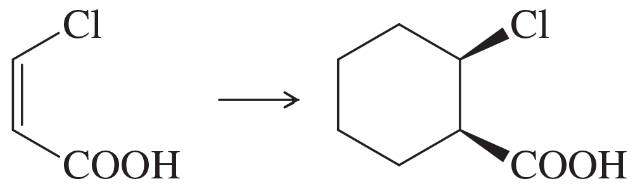
Show how you would accomplish the following syntheses efficiently (you may use any necessary reagents).
(e)
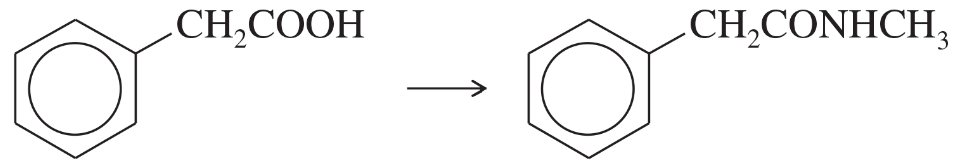
Problem 36f
Show how you would accomplish the following syntheses efficiently (you may use any necessary reagents).
(f)
Problem 36g
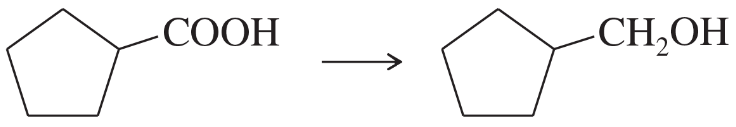
Show how you would accomplish the following syntheses efficiently (you may use any necessary reagents).
(g)
Problem 36h
Show how you would accomplish the following syntheses efficiently (you may use any necessary reagents).
(h)
Problem 37a
Predict the products and propose mechanisms for the following reactions.
(a)