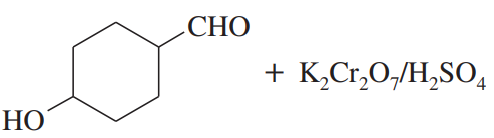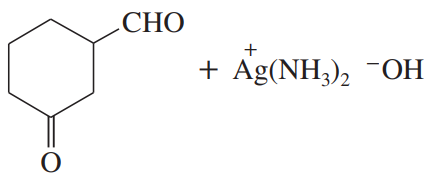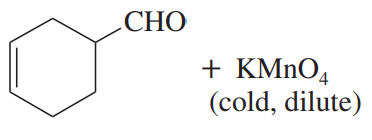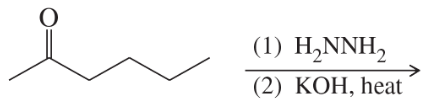Back
BackProblem 31b
Like other strong nucleophiles, triphenylphosphine attacks and opens epoxides. The initial product (a betaine) quickly cyclizes to an oxaphosphetane that collapses to an alkene and triphenylphosphine oxide.
(b) Show how this sequence might be used to convert cis-cyclooctene to trans-cyclooctene.
Problem 32
(a) Outline the syntheses indicated in Solved Problem 18-2, beginning with aldehydes and alkyl halides.
(b) Both of these syntheses of 1-phenylbuta-1,3-diene form the central double bond. Show how you would synthesize this target molecule by forming the terminal double bond.
Problem 33a,b
Show how Wittig reactions might be used to synthesize the following compounds. In each case, start with an alkyl halide and a ketone or an aldehyde.
(a) Ph–CH=C(CH3)2
(b) Ph–C(CH3)=CH2
Problem 33c,d
Show how Wittig reactions might be used to synthesize the following compounds. In each case, start with an alkyl halide and a ketone or an aldehyde.
(c) Ph–CH=CH–CH=CH–Ph
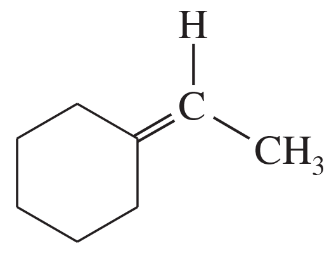
(d)
Problem 34a
Predict the major products of the following reactions.
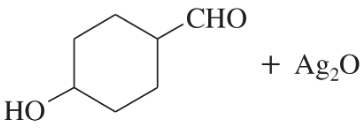
(a)
Problem 34b
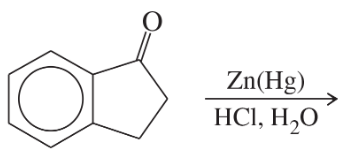
Predict the major products of the following reactions.
(b)
Problem 34c
Predict the major products of the following reactions.
(c)
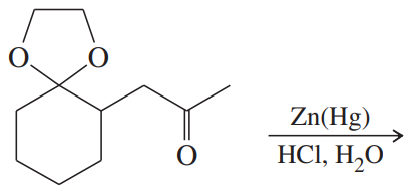
Problem 34d
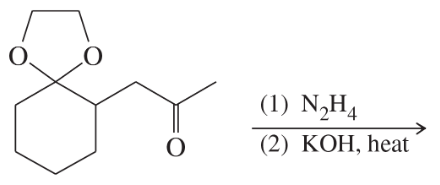
Predict the major products of the following reactions.
(d)
Problem 35a
Propose a mechanism for both parts of the Wolff–Kishner reduction of cyclohexanone: the formation of the hydrazone, and then the base-catalyzed reduction with evolution of nitrogen gas.
Problem 35b
Propose a mechanism for both parts of the Wolff–Kishner reduction of cyclohexanone: the formation of the hydrazone, and then the base-catalyzed reduction with evolution of nitrogen gas.
Problem 36a
Predict the major products of the following reactions:
(a)
Problem 36b
Predict the major products of the following reactions:
(b)
Problem 36c
Predict the major products of the following reactions:
(c)
Problem 36d
Predict the major products of the following reactions:
(d)
Problem 37a
Draw structures of the following derivatives.
(a) the 2,4-dinitrophenylhydrazone of benzaldehyde
Problem 37b
Draw structures of the following derivatives.
(b) the semicarbazone of cyclobutanone
Problem 37c
Draw structures of the following derivatives.
(c) cyclopropanone oxime
Problem 37d
Draw structures of the following derivatives.
(d) the ethylene acetal of hexan-3-one
Problem 37e
Draw structures of the following derivatives.
(e) acetaldehyde dimethyl acetal
Problem 37f
Draw structures of the following derivatives.
(f) the methyl hemiacetal of formaldehyde
Problem 37g
Draw structures of the following derivatives.
(g) the (E) isomer of the ethyl imine of propiophenone
Problem 37h
Draw structures of the following derivatives.
(h) the hemiacetal form of 5-hydroxypentanal
Problem 38b
Name the following ketones and aldehydes. When possible, give both a common name and an IUPAC name.
(b) CH3(CH2)2CO(CH2)2CH3
Problem 38c
Name the following ketones and aldehydes. When possible, give both a common name and an IUPAC name.
(c) CH3(CH2)5CHO
Problem 38d
Name the following ketones and aldehydes. When possible, give both a common name and an IUPAC name.
(d) PhCOPh
Problem 38g
Name the following ketones and aldehydes. When possible, give both a common name and an IUPAC name.
(g) CH3CH2CHBrCH2CH(CH3)CHO
Problem 38h
Name the following ketones and aldehydes. When possible, give both a common name and an IUPAC name.
(h) Ph–CH=CH–CHO
Problem 38j
Name the following ketones and aldehydes. When possible, give both a common name and an IUPAC name.
(j)
Problem 38k
Name the following ketones and aldehydes. When possible, give both a common name and an IUPAC name.
(k)
Problem 38l
Name the following ketones and aldehydes. When possible, give both a common name and an IUPAC name.
(l)